Classification Organic Compounds - Definition, Examples, Structure, Properties - FAQs
Classification of Organic compounds
Classification of organic compounds is mainly based on their structure and based on the functional group present on them. An organic compound contains carbon, hydrogen and oxygen.
What are organic compounds?
Organic compound definition- The organic compounds are classified as the compounds which contain only two chemical elements, one is carbon and other is hydrogen.
Compound meaning in tamil
Compound meaning in tamil is kalavai கலவை, Organic meaning in Tamil is கரிம, Organic compounds meaning in Hindi is कार्बनिक यौगिक
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Organic meaning or Organic chemistry definition and examples.
Organic definition-Organic compounds contain carbon as essential constituents.
Organic chemistry meaning- Organic chemistry is a special branch of chemistry that deals with the study of compounds containing carbon. An organic compound contains carbon and hydrogen. Carbon is an element containing 4 valencies so that it can form 4 bonds with other bonds through covalent bonding. Carbon mostly forms compounds with hydrogen so it is also given the name hydrocarbons. The name organic chemistry is given because all of the carbon-containing compounds were considered to have originated from living organisms. One of the very important definitions of organic chemistry is that it is a branch of chemistry that involves the compounds containing carbon and they are found in living beings.
Organic synonyms
Biological, cellular, living, essential, original, live etc.
Organic matter meaning
Organic matter contains large amounts of carbon compounds present in natural environment.
First synthesized organic compound
The first organic compound synthesized in the laboratory was urea by a German chemist named Friedrich Wohler from ammonium cyanate.
Organic Compounds Examples
Some of the common examples of organic compounds are carbohydrates, liquids, proteins, nucleotides, nucleic acids, alkaloids, nutrients such as vitamin B12, etc. Due to the ability to form a carbon-hydrogen bond is easy there are millions of organic compounds are known. The valency of carbon is 4 and thereby it can form 4 bonds with hydrogen. One of the very common examples is methane. The bond formation in methane is shown below.
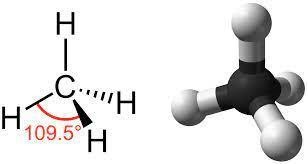
Classification of organic compounds based on structure
There are millions of organic compounds are present so their classification should be in detail also. The classification following is based on the structure.
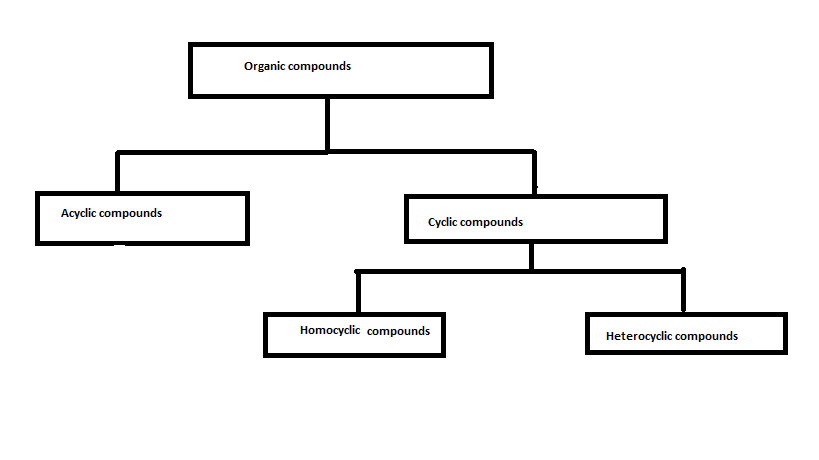
Organic compounds are mainly classified into cyclic compounds and acyclic compounds. The cyclic compounds are again classified into homocyclic and heterocyclic compounds. The homocyclic compounds can be again classified as aromatic compounds and aliphatic compounds. Acyclic compounds or open chain compounds can also be again classified as straight-chain compounds and branched-chain compounds.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
Cyclic Compounds, Ring Compounds or Closed Chain Compounds
The carbon atoms which are joined together to form a ring are known as ring compounds or cyclic compounds. And which is formed when three or more atoms are connected. The very common example is cyclopropane which contains three carbon atoms joined together to form a triangular-like compound. The following figure shows the structure of these compounds.
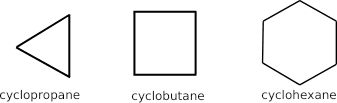
These cyclic compounds are again classified into homocyclic and heterocyclic compounds.
Homocyclic Compounds
When the compounds contain only carbon atoms it is homocyclic. Cyclopropane, cyclobutene, cyclopentane, cyclohexane, etc. are all examples of homocyclic species. It does not contain an element other than carbon atoms. Homocyclic compounds can be further classified into aromatic and aliphatic compounds.
Alicyclic Compounds
These are aliphatic and cyclic compounds. They may be saturated or unsaturated. The bonds present on them are single, double, and triple bonds. Cyclopropane, cyclobutene, etc. are some examples.
Aromatic Compounds
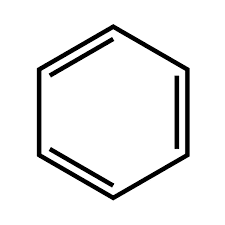
These are cyclic compounds, unlike alicyclic compounds they are unsaturated only. These compounds are called aromatic compounds since they contain a pleasant aroma. Benzene is the most common example. Aromatic compounds possess greater stability due to the presence of conjugated double bonds. It is further classified as benzenoid and non-benzenoid aromatic compounds.
Benzenoid aromatic compounds
These are aromatic compounds that contain one or more benzene rings that may be isolated or fused. Based on the number of rings present it is further classified into monocyclic with one ring, bicyclic with two rings, and tricyclic with three rings. Some of the common examples include naphthalene, anthracene, phenanthrene, etc. The structure of some of these compounds is shown below.
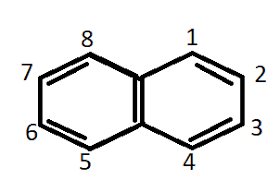
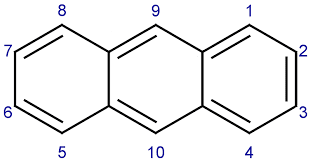
Non-Benzenoid aromatic compounds
These are aromatic compounds that contain unsaturated rings other than benzene. Examples include azulene, oxa azulanones, tropolone, etc.
| Related topics link, |
Heterocyclic compounds classification
These are also cyclic compounds but they contain at least one element other than a carbon atom. Mostly the elements are oxygen, nitrogen, and sulfur. Some of the examples of heterocyclic compounds are tetrahydrofuran, oxirane, thietane, pyridine, pyrrole is examples of heterocyclic species. The structure of some of them is shown below.
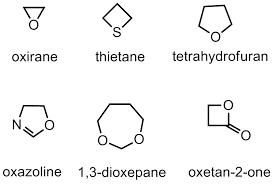
These heterocyclic compounds are further classified into alicyclic heterocyclic compounds and aromatic heterocyclic compounds.
Alicyclic heterocyclic compounds
Heteroatoms like sulfur, oxygen, nitrogen, etc. attached to alicyclic rings are alicyclic heterocyclic compounds. Tetrahydrofuran, tetrahydrothiophene are some examples.
Aromatic heterocyclic compounds
Heteroatoms like sulfur, oxygen, nitrogen, etc. attached to an aromatic ring are aromatic heterocyclic compounds. Thiophene, furan, etc. are some of the examples.
Acyclic or open chain compounds
Here the carbon atoms are arranged in such a way that an open chain or acyclic compound is formed. These are also known as aliphatic compounds. Those chains present on them may be branched or straight chains. Based on that it can be further divided into two types that is straight-chain organic compounds and branched-chain organic compounds.
Straight chain Organic compounds
The carbon atoms arranged in such a way that it forms a straight chain. In naming those compounds the letter ‘n’ is used which represents normal. Some of the examples of which include n-propane, n-butane, n-pentane, etc. When it contains double bonds they are named propene, butene, etc.
Branched-chain organic compounds
For branched-chain organic compounds, their carbon skeleton contains a branched-chain. The name ‘iso’ is used to notate. Some of the examples are isobutane, iso propane, isopentane, etc. When double bonds came it is named as isobutylene, iso propylene, etc.
Classification of organic compounds based on the functional group
Organic compounds can again be classified based on functional group and mainly explains the chemical behavior of organic compounds. The property of organic compounds changes with changing functional groups attached to them. There are many functional groups which include hydroxyl group, carboxyl group, amino group, cyano group, etc. These are explained in detail below given table.
Class | Functional group |
Alcohols | -OH |
Carboxylic acid | -COOH |
Isocynides | -NC |
Ketones | Carbonyl |
Nitro compounds | NO-2 |
Cyanides | Cyano |
Aldehyde | -CHO |
Based on the presence of functional group name, properties, chemical behavior, odor, color, reaction, etc. of organic compounds will change. When the hydroxyl group is present the organic compound will be alcoholic and the ‘ol’ will be used. Examples are propanol, butanol, etc.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
