Diastereomers - Meaning, Example, Properties, Differentiation, FAQs
The stereoisomers that are not related to objects and mirror images are called diastereomers which means that they are not enantiomers. Their mirror images of each other and non-superimposable. Diastereomers are entirely different in many aspects from enantiomers in their physical properties, the reactivity, their melting point, boiling points, densities, etc. are all different. But both the enantiomers and diastereomers are called stereoisomers.
This Story also Contains
- Properties of Diastereomers
- Properties of Diastereomers and Enantiomers
- Diastereomers Example
- Diastereoselectivity
- Diastereomer Meaning
- Superimposable Mirror Images
Properties of Diastereomers
Other than geometrical isomers, diastereomers may or may not be optically active.
Compared to enantiomers they all have a similar physical property except the sign of specific rotation is different but for diastereomers, they have different physical properties such as melting point, boiling point, refractive index, dielectric constant, specific rotation, density, etc. are all different.
As their physical properties are different, diastereomers can be separated from each other through some techniques like fractional crystallization, chromatography, fractional distillation, etc. While enantiomers cannot be separated by any of the above-mentioned techniques. This property can also be utilized in chiral synthesis.
Also read -
Properties of Diastereomers and Enantiomers
When talking about diastereomers and enantiomers tartaric acid, C4H6O6 is a good example. The structure of tartaric acid is very interesting to study also. Tartaric acid is mainly in the form of (R, R). But it can also be in the meso form that is (R, S) by synthesizing artificially. (R, R) tartaric acid is the mirror image to (S, S) tartaric acid, that is they are enantiomeric in nature.
While the meso tartaric acid that is (R, S) is diastereomers to (R, R) tartaric acid. So, (R, R) and (S, S) tartaric acid are enantiomers so they have similar physical properties that their melting point is the same that is 170 degrees Celsius. While the case of meso tartaric acid is different, that is for (R, S) tartaric acid has different physical properties; their melting point is different from other stereoisomers and is about 145 degrees Celsius. The following image shows (R, R), (S, S), and (R, S) of tartaric acid.
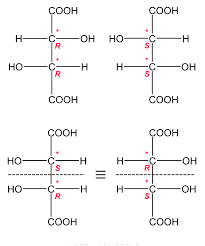
Stereoisomers of tartaric acid
Diastereomers Example
Let us consider another compound possessing two chiral centers 3-Bromo-2-Butanol. From the following structure of 3-Bromo-2-Butanol, we get the facts that one of the configurations is mirror images to each other while the other is non-superimposable and is not mirror images.
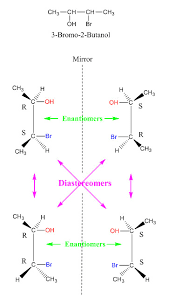
Diastereomers and enantiomers of 3-Bromo-2-Butanol
The (R, S) and (S, R) are enantiomers, (R, R) and (S, S) are enantiomers while (R, S) and (S, S) are diastereomers, and (R, R) and (S, R) are diastereomers.
Therefore, these two configurations are not mirrored images to each other, and also they are not enantiomeric but are diastereomers. From the above-mentioned images, we get a clear-cut picture of diastereomers and also how they differ from enantiomers.
The diastereomers which are different from each other only by one stereocenter having two or more stereocenters are known as epimers. Examples of epimers are D-glucose and D-galactose. They are epimers of each other and as well as diastereomers.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
Diastereoselectivity
For an organic reaction, the preference for the formation of one or more diastereomers is its diastereoselectivity. That is for a chemical reaction when only one diastereomer is preferred over the other diastereomers then the reaction can be called a diastereoselective reaction. This happens when one of the sides is completely blocked by some other compounds. The best example of a diastereoselective reaction is the dehydrohalogenation of 2-iodo-butane. Where this reaction yields both trans and cis product while trans-2-butene is formed in excess that is 60% while cis-2-butene only 20%.
Erythro and Threo Form
Threo and erythro configurations are written when the molecule contains a chiral carbon. Chirality is a property when the mirror image of a molecule is different. If a molecule contains at least one chiral center the molecule is said to be chiral.
For naming molecules with two stereogenic centers erythro and threo forms are commonly used. The following figure tells about the erythro and threo form.
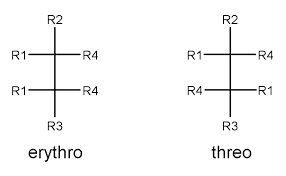
Erythro and Threo Form
| Related topics, |
From the image, we can easily define erythro and threo forms. That is when the same groups R1 and R4 are on the same side it is called erythro form and when the common substituents R1 and R4 are on the opposite side it is called threo form. So we can define erythro form as, when common substituents lie on the same side it is called erythro form while they lie on the opposite side it is called threo form. This approach is mainly used for naming chiral compounds, mainly those having two chiral centers. It is commonly used for naming carbohydrates. The following compound is an example of erythro and threo forms.
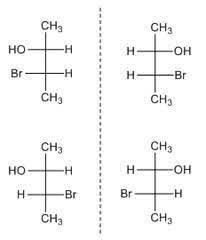
Stereoisomers of 3-Bromo-2-Butanol
For 3-Bromo-2-Butanol when 2 H atoms are present on the same side it is called erythro form (first figure). When 2 H atoms are present on the opposite side it is called threo form (second figure).
For the case of simplicity erythro and threo form nomenclature is replaced with syn and anti stereoisomers that is when two common substituents are present on the same side it is called syn while present on opposite sides of the planes is called anti in a zig-zag carbon skeleton.
Let us consider another example that is 2, 3-dichloroethane. Here two chlorine atoms are present when the two chlorine atoms and hydrogen atoms are present on the same side it is an erythro compound that is erythro-2, 3-dichloroethane. While when two hydrogen atoms and chlorine atoms are present on opposite sides it is a threo compound that is threo-2, 3-dichloroethane. The name erythro and threo are obtained from the names of saccharides that are erythrose and threose. Thus by changing the position of the functional group there will be a great change in their properties. Erythro and threo compounds also have two enantiomeric forms which are represented as D and L enantiomers.
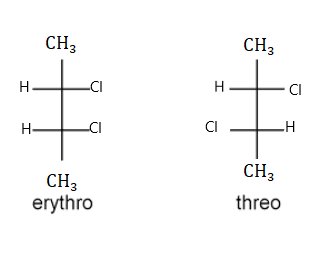
Diastereomer Meaning
Those stereoisomers that do not mirror images and are not superimposable are diastereomers. And they are not enantiomers.
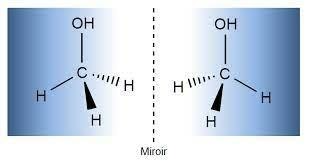
Superimposable Mirror Images
Images that can superimpose are superimposable mirror images. The best example is the right and left hands of humans. Such compounds are called achiral molecules. And those objects and mirror images that cannot be superimposed are non-superimposable mirror images and are chiral compounds. The following is an example of a non-superimposable mirror image.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
