Diazotization Reaction Mechanism - Detailed Information with FAQs
Diazotation is a Procedure of Generating Diazonium Compounds or Diazonium Salts.
This reaction was first illustrated by Peter Griess. Hence, the diazotization technique yields salts of aromatic compounds through aromatic amines.
Diazotization Reaction:-
Diazotization Reaction involves the formation of diazonium salts when aromatic amines are made to react with nitrous acid in presence of mineral acid. Water is obtained as a by-product or side product.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Diazotation is a Procedure of Generating Diazonium Compounds or Diazonium Salts.
- Diazotization Mechanism:-
- Reaction is illustrated as follows:-
- Diazotization Titration:-
The reaction can be clarified as follows:-
Aromatic Amine + Nitrous Acid + Mineral Acid gives Diazonium Salt + Water
Aromatic amine is usually a derivative of aromatic hydrocarbon where the hydrogen of the aromatic ring is replaced by or substituted by an amino group. This amine group is nucleophilic and directly bonded to the aromatic ring compound. Nitrous acid is generally a nitrogen oxoacid a conjugate acid of nitrite. It is a monoprotic weak acid used in the gaseous phase. The structure of nitrous acid is as follows-
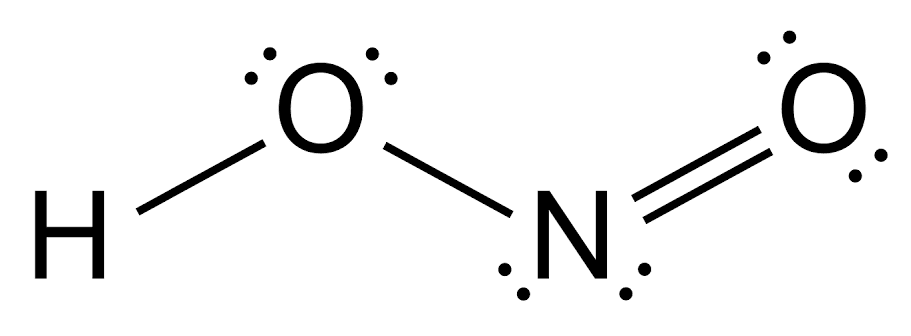
Mineral acids or inorganic acids are derived from one or more inorganic chemical compounds. Mineral acids when dissolved in water tend to produce hydrogen ions and conjugate base.
General diazotization reaction is as follows:-
ArNH2 + HNO2 + HX ---------- ArN2+X- + H2O
Diazotization of an Amine:
Diazotization of amines is a process where primary amines are converted to diazonium salts using nitrous acid and mineral acid respectively.
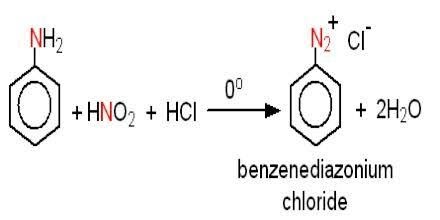
This reaction involves losing nitrogen from the aromatic amine generating a carbon cation further leading to the formation of a diazonium salt.
Diazotization Mechanism:-
Now, let us have a look at the mechanism followed by aromatic primary amine to form diazonium salt.
Formation of nitrosonium ion
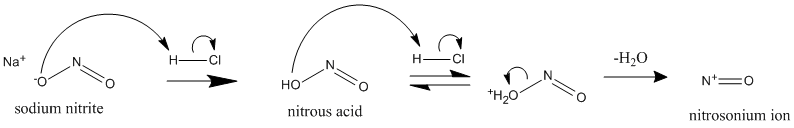
The main reagent used in this reaction is either sodium
nitrite (NaNO2) or Nitrous acid (HNO2).
Sodium nitrite salt can be easily handled while Nitrous acid is an unstable liquid to be handled cautiously.
Secondly, mineral acid plays an important role in converting NaNO2 to HNO2 provided if NaNO2 is used in the reaction.
The mineral acid generally used is HCl.
HCl converts NaNO2 to HNO2 further converting it into nitrosonium ion (NO+) which is a strong electrophile.
This electrophile is responsible for the formation of a diazonium salt. The formation of nitrosonium ion takes place by protonation of a hydroxyl group (OH-) resulting in loss of a water molecule.
Formation of diazonium ion
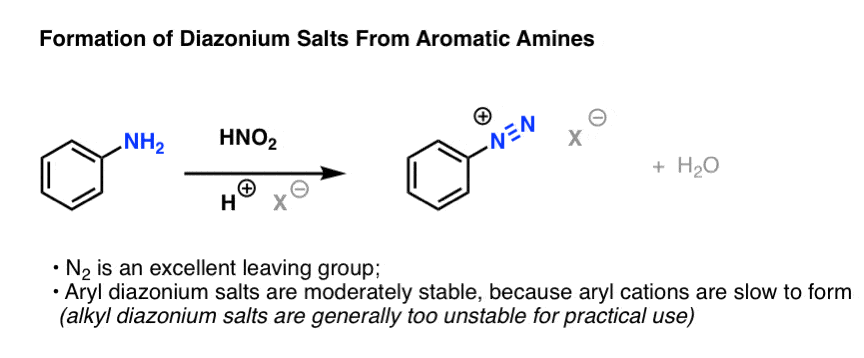
The reaction between amine and nitrosonium ion
accompanied by mineral acid is the next step.
The reaction between nitrosonium ion and aromatic amine leads to the formation of a new N-N bond.
Two proton transfers take place from nitrogen to oxygen followed by rearrangement of pi bonds.
i.e. N-N a pi bond is formed and an N-O bond is broken.
N-N triple bond is formed with the elimination of water molecule.
This is the final step in the diazotization reaction mechanism.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 Haloalkanes and Haloarenes
- NCERT notes Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
Diazonium Coupling Reaction:-
We all know that dyes form an important part of our day to day lives. Dyes are nothing but diazobenzene derivatives. The structure of diazobenzene or azobenzene is such that the two benzene molecules are attached by the nitrogen-nitrogen double bond. The most important characteristic of diazonium salts is that they undergo coupling reactions. The azo group is the N-N bond present in the compound.
Aromatic diazo compounds are stable and crystalline chemical species. They can be synthesized by azo cooling reaction following electrophilic substitution reaction. The reaction takes place between the aryl diazonium salt and an aryl ring. The aryl ring attacks the diazonium cation forming azo compounds.
ArN2+ + Ar'H ------------ ArN=NAr' +H+
The diazo coupling chemical reactions take place at or near 0°C as the diazonium compounds or diazonium salts are unstable at the above-mentioned temperature.
The azo compound formation also takes place via the oxidation of hydrazines (R-NH-NH-R).
Condensation of nitro aromatic compounds with aniline result in the formation of azoxy intermediate which is further reduced to the azo compound.
Ar(NO2) + ArNH2 ArN(O)=NAr + H2O
ArN(O)=NAr + C6H12O6 ArN=NAr +
C6H12O6 + H2O
As a result of π-delocalisation of electrons, Aromatic diazo compounds express themselves in various colours, mostly red, orange and yellow. Hence, they are usually used as dyes and so are called azo dyes.
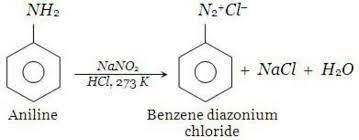
Diazotization Of Aniline:-
Aniline or Arenediazonium salt reacts with a highly reactive compound like phenol and or amines leading to the formation of coloured compounds called azo compounds. This reaction is termed a coupling reaction. This chemical reaction is used in the preparation of red azo dye. Preparation of aromatic diazonium salt generally takes place by the addition of cold aqueous sodium nitrite to primary aromatic amine in presence of an acid. The temperature required for this reaction to take place is 273 – 278K.
Related Topics Link, |
Reaction is illustrated as follows:-
Aliphatic Azo or Diazo compounds are less used than aromatic diazo compounds. Aliphatic azo or diazo compounds are used as radical initiators. Diethyldiazine, an aliphatic azo compound cleaves the C-N bond at increased temperature leading to the elimination of nitrogen gas generating radicals.
The aliphatic azo chemical should be handled with great care as they can explode due to their instability.
NCERT Chemistry Notes:
Diazotization Titration:-
Converting primary amine the diazonium salt or compound is called diazotization titration.
A primary amine is made to react with sodium nitrite and mineral acid like HCl to obtain a diazonium salt compound.
Nitrous acid is formed when sodium nitrite and HCl react with each other.
NaNO2 + HCl →NaCl + H2O
The nitrous acid forms react with primary amine forming diazonium salt. The addition of ammonium sulphamate solution is added to remove excess nitrous acid formed.
R-NH2 + HNO2→ R-N=NH + H2O
Starch iodide paper is used in the detection of the endpoint. The end point is detected by formation of blue colour.
The starch iodide paper preparation involves immersion of filter paper in starch and potassium iodide solution.
The following reactions depict the end point raections:-
KI + HCl→ KCl + H2O
2HI + 2HNO2 →I2 + 2NO=2H2O
I2 + starch mucilage →blue colour
Also check-
Frequently Asked Questions (FAQs)
Formation of diazonium salts through primary aromatic
amines is termed as diazotizaton reaction.
Diazotization reaction and azo coupling reactions require
low temperature as these diazonium salts form other
compounds at elevated temperature and provide
phenol by reacting at increased temperature.
Aromatic diazonium compounds or salts are unstable
and form hydrogen chloride (HCl), nitrogen gas (N2),
and chlorobenzene.
Absence of conjugation leads to the unstability of
aliphatic diazonium salts whereas a benzene ring is
present in aromatic diazonium salt which accounts
stability of the compound due to the presence of
conjugation.
Diazonium salts are used in the production of organic compounds.
Three main types of diazotization titration are as
follows:
Direct method-
It involves the treatment of a drug containing amino group with acid.
Indirect method-
This method is mainly used for the titration of insoluble diazonium salts or compounds. Excess of HNO2 is added to the titration species and is back titrated with the required titrant.
Other method-
This method involves the production of diazo oxide rather than diazo compound as diazo oxide is more stable.
Also Read
13 Dec'24 09:35 AM
12 Dec'24 04:35 PM
13 Nov'24 05:00 PM
18 Oct'24 11:58 AM
30 Sep'24 08:52 AM
17 Jun'22 05:48 PM
17 Jun'22 04:12 PM
17 Jun'22 04:06 PM
16 Jun'22 06:51 PM