Red Phosphorus - Structure, Properties, Sources, Uses, FAQs
Introduction
The chemical element with atomic number 15 and generally recognized with the symbol P, is known as phosphorus.
Henning Brandt in 1669 at Hamburg, invented phosphorus, by evaporating animal urine and heating the residue till it was red hot, after which the vapors of phosphorus distilled, which Henning collected by condensing it in aqueous medium.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs
- Red phosphorus
- Structure of red phosphorus
- Properties of red phosphorus
- Red phosphorus uses
Henning kept the invention confidential, thinking he discovered the stone which turns base metal into gold called the Philosopher’s stone. After Brandt ran out of capital, he sold his discovery of phosphorus to Daniel Kraft who exhibited it in Europe and London which grabbed Robert Boyle’s attention and fascinated him. Robert Boyle investigated the discovery of phosphorus and started producing it systematically.
Phosphorus composition
Phosphorus is mainly composed of the phosphate ion, PO3-4. It is a P block element, found in group 15, period 3 in the modern periodic table, with electronic configuration [Ne] 3s2 3p3.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Appearance of phosphorus
Phosphorus is metallic looking and is waxy in nature.
Phosphorus color
The color of phosphorus is white, yellow, red, violet, and black with metallic luster.
Allotropes of phosphorus
The two main allotropes of phosphorus are white phosphorus and red phosphorus. Red phosphorus is amorphous in nature and is non toxic solid, whereas white phosphorus is waxy and is poisonous, which after coming in contact with skin causes severe burns. White phosphorus is luminescence in nature, also known as phosphorescence and also catches fire when exposed to air.
Red phosphorus
Red phosphorus formula is P4.
As stated above, red phosphorus is a stable and nontoxic allotrope of phosphorus and it is a P4 molecule derivative. Red phosphorus as the name suggests, is red in color, it is odor free and less reactive in nature than white phosphorus. Unlike white phosphorus, red phosphorus does not have the property of phosphorescence.
Discovery of red phosphorus
Red phosphorus was invented by Australian chemist Anton Von S in the year 1844. Anton ignited the white phosphorus up to the temperature of 428o F with nitrogen for a few hours and the product produced was red phosphorus.
Structure of red phosphorus
The structure of red phosphorus is polymeric, consisting of 4 tetrahedrally grouped phosphorus atoms which are bonded together, in which one of the P-P bond is broken down and one extra bond is formed with the neighboring tetrahedron giving out an structure which is chain like in appearance.
Red phosphorus structure is shown below.
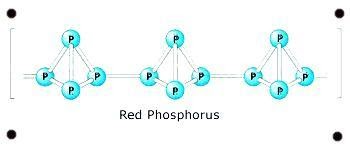
Properties of red phosphorus
- Red phosphorus is red in color
- Molar mass is 30.974 g/mol.
- Physical constant is 860K.
- It is amorphous in nature.
- It is odor free.
- Its density is 2.34 g/cm3.
- It is nontoxic and less reactive than white phosphorus.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 7 The P-block elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 7 The P-block elements
- NCERT notes Class 12 Chemistry Chapter 7 The P-block elements
Red phosphorus method
White phosphorus is used for extracting red phosphorus. It is an intermediate product of violet and white phosphorus, the sole reason why most of its properties have a range of values.
For example, freshly made red phosphorus is highly reactive in nature with an ignition temperature of 300oC which after storing or continuous heating, its red color darkens causing it to become less reactive in nature.
Red phosphorus is made by heating white phosphorus at a temperature of 482oF in presence of nitrogen and iodine as catalysts.
Another method of preparation of red phosphorus is from bone ash
Firstly, take a quantity of finely ground powder from rocks that are quite rich in animal or fish bones or phosphorus directly. Then treat this bone ash with sulfuric acid, which after treatment yields calcium sulfate as the minor product and phosphoric acid as the major product. After getting phosphoric acid heat it with charcoal which yields white phosphorus, which on further heating yields red phosphorus.
Sources of red phosphorus
- From urine: common source of phosphorus is animal urine which Brandt used for the first time for the isolation of phosphorus, he purified the urine, boiled it until it turned into a paste on a high temperature, distilled the vapors and condensed it into white phosphorus, which on further heating degraded the white phosphorus into red phosphorus. It is a time taking process.
- From rocks or bone ash: this method is a somewhat ancient method used in the 17th century, which produces phosphorus from rocks or bone ash. Firstly, take a reduced form of animal bones by finely grinding phosphorus rich rocks for example, pyromorphite, which on treatment with sulfuric acid yields phosphoric acid as major product and calcium sulphate as minor product. The phosphoric acid obtained on treatment with charcoal over flame gives white phosphorus, which on further heating gives red phosphorus.
- Other tertiary sources: phosphorus is an important constituent of human body which helps in formation of bones and teeth, it is also a requirement for the human body to manufacture proteins and it is also an important component in the formation of adenosine triphosphate (ATP), which is the energy source for normal working of human body. The kidney gives out extra phosphorus through urine, which is why urine is used as a good source for extraction of phosphorus.
| Related topics link, |
Red phosphorus uses
- It is used for producing fertilizers and pyrotechnics.
- It is used in matchsticks which are used for lighting a fire.
- It is used for production of electroluminescent coating and semiconductors.
- It is also used as intermediate in organic synthesis.
- It is also used in smoke bombs because of its reactive and stable nature.
- Few components of red phosphorus are used in softening of water as well.
- The flares of red phosphorus are used in emergency signals to help in the ignition process.
Red phosphorus with water
In the Breaking bad tv series, it is shown that the character makes phosphine gas by dropping red phosphorus in hot water but in reality, it’s a myth. Red phosphorus does not ignite in hot water, nor does hydrolysis take place, neither creates an explosion.
Red phosphorus is chemically
Red phosphorus is believed to have a long chain-like structure of tetrahedron of P4, that’s the reason it is polymerized highly and thus it is chemically unreactive.
Images of red phosphorus
Phosphorus images are shown below.



Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Austrian chemist Anton Von S in 1844 invented red phosphorus.
P4 (long chain)
Red phosphorus can be prepared using white phosphorus obtained from phosphorus from animal urine and second by bone ash method.
Ca5 (OH) (PO4)3
The ignition temperature of white phosphorus is 30oC (86oF).
Beans, lentils, chicken and pork meat, whole grain breads, green leafy vegetables and fruits are rich sources of phosphorus.
For adults up to 18 years of age it is 4,700 mg/day and for children and teens in the age group between 4,500 to 4,700 mg/day.
Red phosphorus is amorphous solid and is nontoxic, odour free, less reactive and stable in nature whereas white phosphorus is waxy, more reactive, phosphorescence in nature. Red phosphorus has wider applications than white phosphorus because of its less reactive and stable nature.
Also Read
09 Dec'24 10:53 AM
30 Sep'24 11:36 AM
30 Sep'24 11:34 AM
30 Sep'24 11:34 AM
30 Sep'24 11:30 AM
30 Sep'24 10:59 AM