ATP and NADPH Full Form
What is the full form of ATP and NADPH?
The full form of ATP is ‘Adenosine Triphosphate’, while NADPH refers to “Nicotinamide Adenine Dinucleotide Phosphate”
What is ATP?
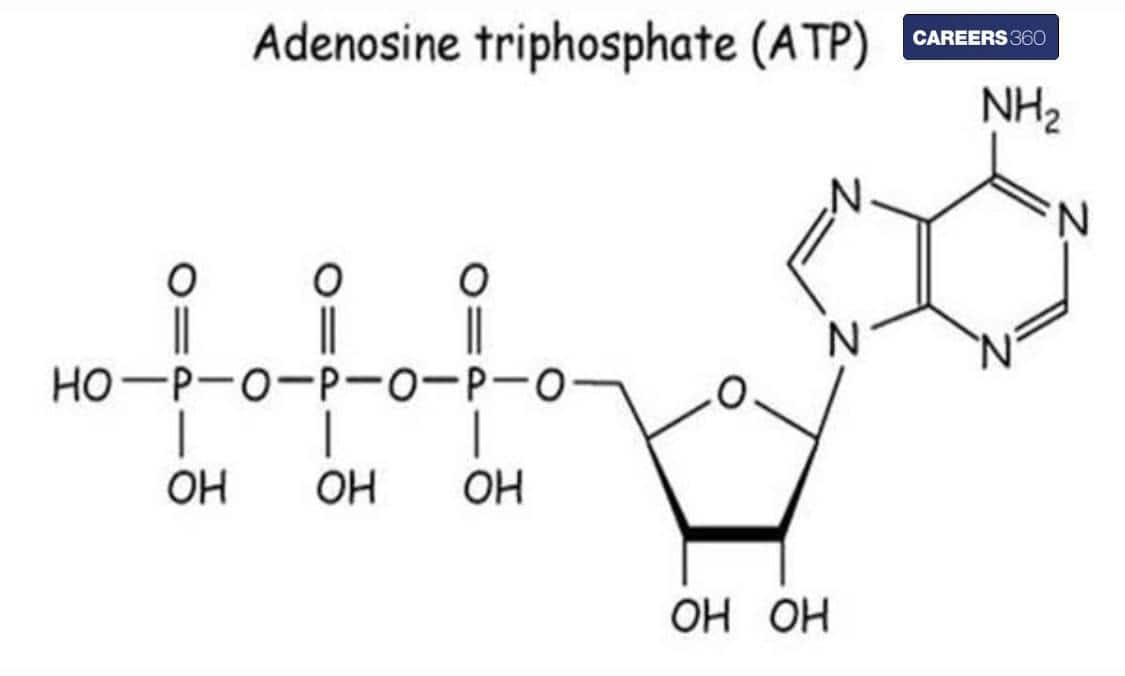
ATP, that is ‘Adenosine Triphosphate’, is the source of energy that the cells consume and store. Adenine, ribose, and three serially bound phosphate groups make up the structure of ATP, which is a nucleoside triphosphate. It is a crucial "energy molecule" that all biological forms contain. It supplies energy to power a variety of biological functions, including chemical production, condensate dissolution, nerve impulse transmission, and muscular contraction.
What is NADPH?
NADPH, that is ‘Nicotinamide Adenine Dinucleotide Phosphate’, it is a cofactor used in anabolic processes that need NADPH as a reducing agent ('hydrogen source'), such as the Calvin cycle and the synthesis of lipids and nucleic acids. All types of cellular life use it. The diminished version of NADP+ is NADPH. The pentose phosphate pathway, which is activated in the first stage by glucose-6-phosphate dehydrogenase (G6PDH), is the main source of NADPH in mammals and other non-photosynthetic organisms.
Chemical properties of ATP
In the absence of catalysts, ATP is stable in aqueous solutions with a pH range of 6.8 to 7.4. It quickly hydrolyzes to ADP and phosphate at pH levels that are more severe. When compared to equilibrium, the ratio of ATP to ADP is maintained in living cells at a point where ATP concentrations are five times higher than those of ADP.
Chemical properties of NADPH
NADPH can also be produced by methods unrelated to the metabolism of carbon. One such instance is ferredoxin reductase. Nicotinamide nucleotide transhydrogenase, which is present in many bacteria and eukaryotic mitochondria, transfers hydrogen between NAD(P)H and NAD(P)+. There are variations that function without a proton gradient and versions that do. Using NADP-linked hydrogenase, some anaerobic organisms break apart a hydride from hydrogen gas to create a proton and NADPH.
Functions of ATP
The transport of various molecules across cell membranes is one of the many cellular processes for which ATP is consumed.
Providing the energy necessary for muscular contraction, blood circulation, movement, and other bodily functions are among ATP's additional roles.
An important function of ATP outside of energy production is the synthesis of the thousands of different macromolecules needed by cells to survive. The ATP molecule is also used as a switch to regulate chemical processes and to transmit signals.
Functions of NADPH
All organisms depend on the electron donor nicotinamide adenine dinucleotide phosphate (NADPH), which also supplies the reducing force for anabolic processes and redox balance. Numerous metabolic enzymes and several signal transduction pathways, which go through adaptive modification in cancer cells, control NADPH homeostasis.
Structure and Formula for ATP
The structure is given below.
The chemical formula for ATP is C10H16N5O13P3.
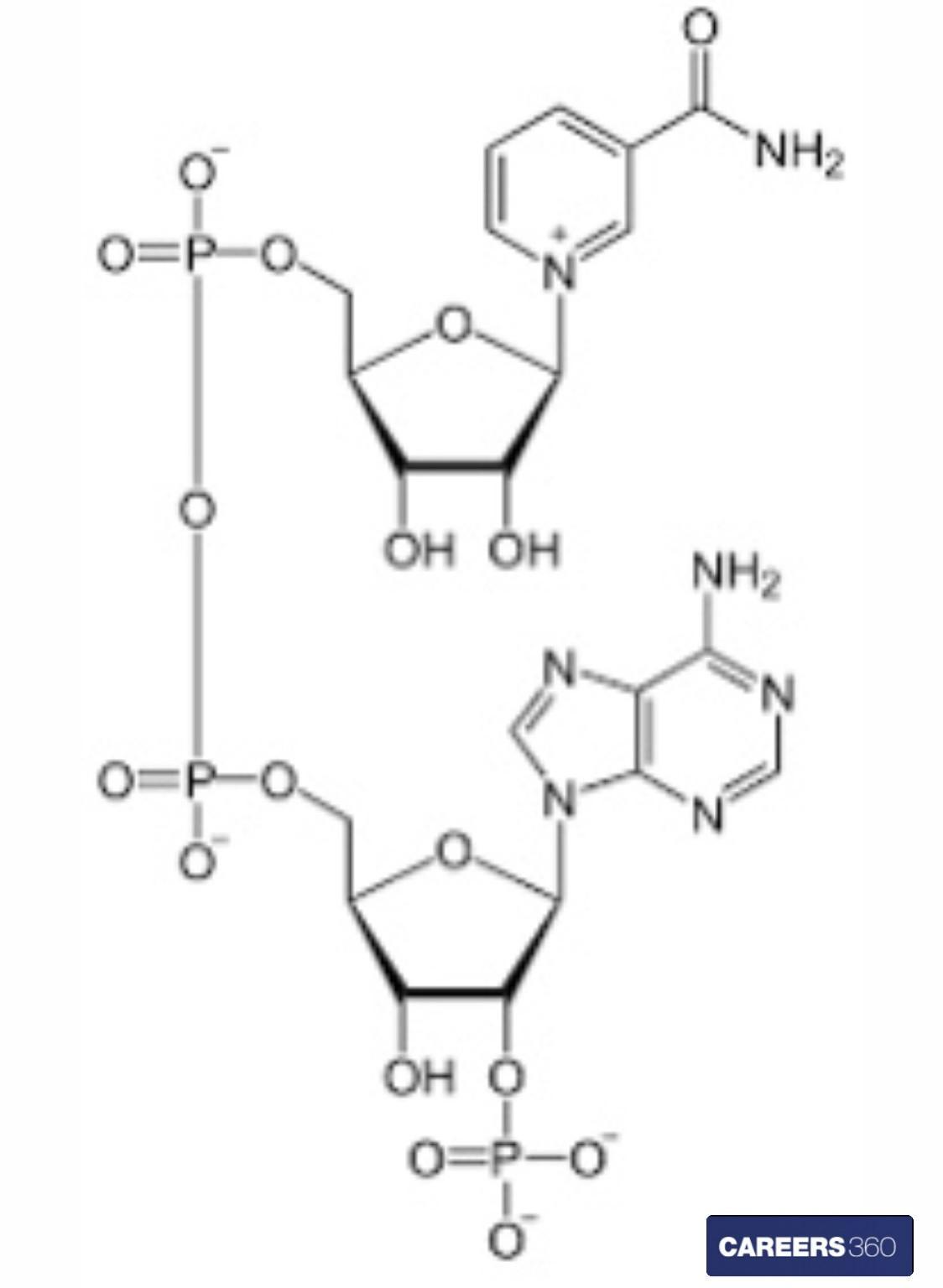
Structure of NADPH
The chemical structure of NADPH is given below.
Frequently Asked Questions (FAQs)
Full form of ATP is Adenosine Triphosphate.
Full form of NADPH is Nicotinamide Adenine Dinucleotide Phosphate.
a) The primary energy unit of the cell is ATP.
The cell's primary reducing agent is NADPH.
b) ATP hydrolysis releases the energy required by the majority of the cell's metabolic processes while NADPH gives hydrogen atoms and electrons to biological reactions.
ATP has a molar mass of 507.18 g/mol.
NADPH has a molar mass of 744.413 g/mol.
The light response of photosynthesis results in the production of ATP and NADPH. The biosynthetic pathway employs these. Additionally, they are employed in the Calvin cycle to lower carbon dioxide. The carbon dioxide is converted to sugar in that process.
