Alcoholic Fermentation: Definition, Equation, Process, Steps, Uses
Alcoholic fermentation is an anaerobic breakdown of sugars by yeast to produce ethanol and CO₂, allowing glycolysis to continue when oxygen is absent. It is widely used in brewing, winemaking, baking, and certain industrial processes where yeast metabolizes glucose into alcohol. This pathway is a key NEET concept linking glycolysis to anaerobic ATP production.
This Story also Contains
- What Is Alcoholic Fermentation?
- Organism Responsible for Alcoholic Fermentation
- Chemical Equation of Alcoholic Fermentation
- Steps of Alcoholic Fermentation (2-Step Pathway)
- Products of Alcoholic Fermentation
- Why Alcoholic Fermentation Slows or Stops
- Alcoholic Fermentation vs Lactic Acid Fermentation
- Role of Glycolysis in Alcoholic Fermentation
- Importance & Industrial Applications
- Alcoholic Fermentation NEET MCQs (With Answers & Explanations)
- Recommended video on Alcoholic Fermentation

What Is Alcoholic Fermentation?
Alcoholic fermentation is an anaerobic process where sugars are transformed into ethanol and carbon dioxide. This process takes place within the yeast cells' cytoplasm, specifically for Saccharomyces cerevisiae, which multiply in a low-oxygen environment. Even in the presence of oxygen, yeast can use fermentation instead of aerobic respiration in the presence of a high sugar concentration.
It is an age-old technique that has been utilized for several millennia in brewing, the manufacture of wine, and baking—the ingenuity of microorganisms in transmuting simple raw materials into pleasurable products. The study of alcoholic fermentation, therefore, does not only give meaning to this principal food processing method but most importantly to the various biochemical processes it encompasses.
Organism Responsible for Alcoholic Fermentation
The primary agent of alcoholic fermentation is the yeast, Saccharomyces cerevisiae. This species is favoured in fermentation industries due to its efficiency in converting sugars into ethanol and carbon dioxide. In optimal conditions, S. cerevisiae will dominate the fermentation, producing desirable flavours and aromas in alcoholic beverages.
Chemical Equation of Alcoholic Fermentation
The chemical equation for alcoholic fermentation can be summarized as: C6H12O6 → 2C2H5OH + 2CO2
This equation thus shows that indeed one glucose molecule is converted into two ethanol molecules and two carbon dioxide molecules, with a net gain of two ATP molecules.
Steps of Alcoholic Fermentation (2-Step Pathway)
The two steps of alcoholic fermentation are:
Step 1 — Pyruvate Decarboxylation
Enzyme: Pyruvate decarboxylase
Pyruvate is converted into acetaldehyde and carbon dioxide
CO2 forms bubbles in breads and beverages
Step 2 — Reduction of Acetaldehyde
Enzyme: Alcohol dehydrogenase
Acetaldehyde and NADH is converted into ethanol and NAD+
NAD+ is restored and needed for glycolysis
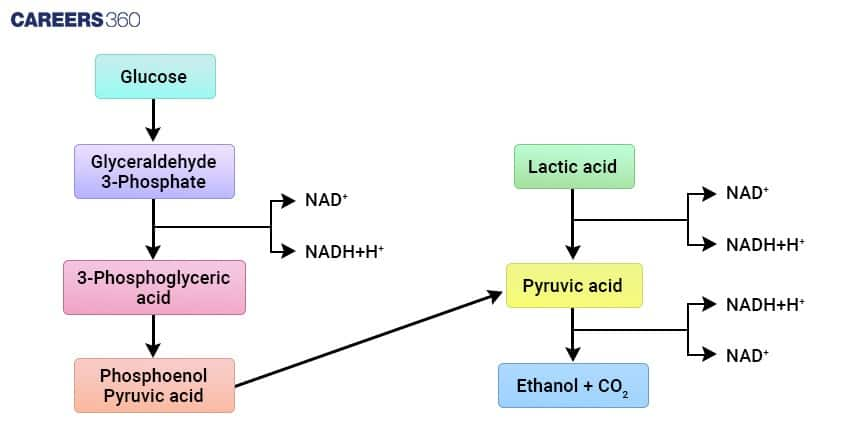
Products of Alcoholic Fermentation
The two primary products are ethanol and carbon dioxide. Ethanol is the primary product recognizable in ethyl product manufacture, while carbon dioxide provides carbonation in beverages and causes bread to rise.
Also generated along with these two primary products are several other products that result from the fermentation process and lead to more multi-faceted flavours in food products.
Secondary Flavor Compounds
Besides ethanol and carbon dioxide, the by-products of alcoholic fermentation are complex and consist of many components that give the sensory attributes to the fermented products. These include:
Acetic Acid: causes sharp character in taste.
Diacetyl: forms buttermilk or buttery smell.
Glycerol: gives body and sweetness
Higher Alcohols: gives the complexity of the flavour.
Esters: gives fruitiness in the aroma.
Succinic Acid: gives the general flavour.
Why Alcoholic Fermentation Slows or Stops
Towards the end, or even in some cases the rate of fermentation reduces to almost zero because of the following reasons:
Nutrient Deprivation: The level of some of the nutrients may drop too low.
Alcohol Toxicity: High ethanol levels turn off yeast activity.
Temperature Extremes: Temperature changes will stress yeast cells.
Oxygen Exposure: Any oxygen can ruin fermentation.
These factors may cause incomplete fermentation, and intervention is needed to get the fermentation going again or to prevent spoilage.
Alcoholic Fermentation vs Lactic Acid Fermentation
The difference between alcoholic fermentation and lactic acid fermentation are:
Feature | Alcoholic Fermentation | Lactic Acid Fermentation |
Organism | Yeast | Bacterial, muscles |
Products | Ethanol and CO2 | Lactic acid |
CO2 evolution | Yes | No |
ATP | 2 ATP | 2 ATP |
Key enzymes | Pyruvate decarboxylase, Alcohol dehydrogenase | Lactate dehydrogenase |
Role of Glycolysis in Alcoholic Fermentation
Glycolysis is the initial stage in alcoholic fermentation. In glycolysis, one glucose molecule gets converted to two pyruvate molecules with a net gain of two ATP and NADH molecules. Under anaerobic conditions, NADH cannot be oxidised in ETC and yeast reduces acetaldehyde to regenerate NAD+. Without fermentation glycolysis stops.
Importance & Industrial Applications
The significant applications of alcoholic fermentation are in the food industry, particularly in brewing.
Brewing
Wine making
Making bread
Production of biofuel like bioethanol
Flavor industries
Alcoholic Fermentation NEET MCQs (With Answers & Explanations)
Important topics for NEET are:
Steps of alcoholic fermentation
Lactic acid vs Alcoholic fermentation
Practice Questions for NEET
Q1. The most abundant prokaryotes helpful to humans in making curd from milk and in production of antibiotics are the ones categorised as:
Cynabacteria
Archaebacteria
Chemosynthetic autotrophs
Heterotrophic bacteria
Correct answer: 4) Heterotrophic bacteria
Explanation:
The most abundant prokaryotes helpful to humans in making curd from milk and in the production of antibiotics are heterotrophic bacteria. Heterotrophic bacteria are useful to humans in a variety of ways. But they are also known to cause various harmful diseases.
Hence, the correct answer is option 4) Heterotrophic bacteria.
Q2. What amount of energy is released from glucose during lactic acid fermentation?
More than 18%
About 10%
Less than 7%
Approximately 15%
Correct answer: 3) Less than 7%
Explanation:
Lactic acid fermentation is an anaerobic (oxygen-free) process that converts glucose to lactic acid and ATP, which is the energy source. During aerobic respiration, 38 ATP are released per glucose molecule. During lactic acid fermentation, less than 7 % of the total energy in glucose During alcoholic fermentation, 2 ATP is released per glucose molecule.
Hence, the correct answer is option 3) Less than 7%.
Q3. What type of reaction is lactic acid fermentation?
Oxidation
Reduction
Hydrolysis
Dehydration
Correct answer: 2) Reduction
Explanation:
Lactic acid fermentation is a critical anaerobic process that transpires in the absence of oxygen, wherein pyruvate, a glycolysis byproduct, is transformed into lactic acid or lactate. This biochemical event exemplifies a redox reaction, during which NADH is oxidized back to NAD⁺, a pivotal step because it facilitates the persistent production of ATP via glycolysis despite oxygen scarcity. The essential chemical transformation is succinctly represented as:
Pyruvate + NADH ⇌ Lactic acid + NAD⁺
This mechanism is frequently observed in muscle cells during rigorous physical activities when the oxygen supply is inadequate to meet the cell's energy demands. Additionally, certain microbes, such as lactic acid bacteria, also exhibit this fermentation pathway. The significance of regenerating NAD⁺ lies in its role as an electron acceptor in glycolysis, thus ensuring the continuation of the energy-yielding process even in anaerobic conditions. Consequently, this process is vital for organisms that encounter environments lacking in oxygen, such as muscle cells during intense exercise and the aforementioned bacterial species.
Hence, the correct answer is option 3) Reduction.
Also Read:
Recommended video on Alcoholic Fermentation
Frequently Asked Questions (FAQs)
These can be due to the exhaustion of nutrients, high alcohol concentration, extreme temperature, and exposure to oxygen, whereby the fermentation slows down or even stops.
Alcoholic fermentation is in common use in the manufacture of beer, wine, and spirits, and in baking for raising bread.
Regeneration of NAD+ for glycolysis so that the yeast can continue to make ATP in anaerobic conditions.
Thousands of years in which ancient civilizations used it in the production of beer and wine.
So, even if oxygen is present, the yeast can still undertake fermentation instead of aerobic respiration if there is too much sugar.