Ammonification - Overview, Examples, Defination
Ammonification is the stage of the nitrogen cycle that contributes to soil fertility and other environmental processes. The process of ammonification forms a link in the recycling of nitrogenous compounds. This topic is important for students pursuing a biology course, especially those appearing for entrance exams like NEET, AIIMS, nursing and courses of paramedical. The ammonification process is defined in the chapter "Mineral Nutrition" of Class 11 CBSE Biology.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
NEET Important PYQ's Subject wise: Physics | Chemistry | Biology
New: Meet Careers360 B.Tech/NEET Experts in your City | Book your Seat now
- Ammonification Definition
- Meaning Of Ammonification
- Ammonification In Nitrogen Cycle
- Process Of Ammonification
- Importance Of Ammonification
- Example Of Ammonification
- Ammonification vs. Other Processes In The Nitrogen Cycle
- Tips, Tricks, And Strategies For Studying Ammonification
- Weightage Of Questions On Ammonification In Exams
- Types Of Questions Asked On Ammonification In Exams
- Recommended Video On Ammonification
Also Read:
- Nitrogen Metabolism
- Mineral Toxicity
- Minerals: Definition, Flow Chart, Benefits
- Essential Mineral Elements
Ammonification Definition
Ammonification is the process by which decomposers like bacteria and fungi convert dead organic nitrogen into ammonia (NH3) or ammonium ions (NH4⁺). This process is vital in the nitrogen cycle because it helps ensure that nutrients are recycled back into a form available to plants.
Meaning Of Ammonification
Ammonification can be defined simply as the breakdown of organic nitrogen into ammonia, which reintroduces the nitrogen in the soil for further absorption by plants.
Ammonification In Nitrogen Cycle
The other critical step in this cycle of nitrogen is ammonification. It is the process of movement of nitrogen between the atmosphere, soil, and organisms through biogeochemical cycles. The absence of ammonification would mean an inability to use the nitrogen trapped in organic matter for growing plants and, hence, breaking ecosystems.
Process Of Ammonification
The ammonification process begins with dead plant and animal matter, and excreted wastes. Here is the procedure divided into individual steps:
Step 1: Decomposition Of Organic Nitrogen
- When plants and animals die, decomposer organisms, mostly bacteria and fungi, feed on the dead organic matter.
- These organisms metabolize nitrogen-containing compounds such as proteins, nucleic acids, and urea, whereby they release ammonia (NH₃) or ammonium ions (NH₄⁺).
Step 2: Conversion To Ammonia (NH₃)
Decomposer bacteria convert organic nitrogen compounds to ammonia.
By undergoing enzymatic reactions, the chemical equation for ammonification is as follows:
Proteins + Water through enzyme activity → Amino acids
Amino acids through microbial action → Ammonia (NH₃) or Ammonium ions (NH₄⁺)
Step 3: Emission Of Ammonia
Once the ammonia is formed, it may stay as ammonium ions (NH₄⁺) in the soil if the soil is acidic or it diffuses into the atmosphere as ammonia gas (NH₃).
Ammonification Reaction
A simple equation can be represented as:
Organic Nitrogen (Proteins/Dead Material) → Bacteria → Ammonia (NH₃) + Water (H2O)
Role In Nitrogen Availability
Ammonium ions (NH₄⁺) are either absorbed directly or further processed through nitrification, converting it to nitrates (NO₃⁻), a readily available source of nitrogen for plants.
Importance Of Ammonification
It is crucial for the overall health and fertility of soil. It is involved in recycling nitrogen from dead organic matter that otherwise remains locked and unavailable for plant growth. It also keeps the nitrogen cycle running, a necessity to keep ecosystems balanced.
Role In Agriculture
- Ammonification replenishes the nitrogen source in soil, which is essential for plant growth and crop production improvement.
- Organic fertilizers rely on the process of ammonification to replenish the nitrogen source in soil throughout various agricultural farms.
Example Of Ammonification
A good example of ammonification in action is in composting. Organic wastes such as decaying plant material, kitchen wastes, and manure from animals decompose with ammonification. This converts the nitrogen of these materials into ammonia which can then be absorbed by the plants for growth improving soil fertility.
Diagram Of Ammonification In The Nitrogen Cycle
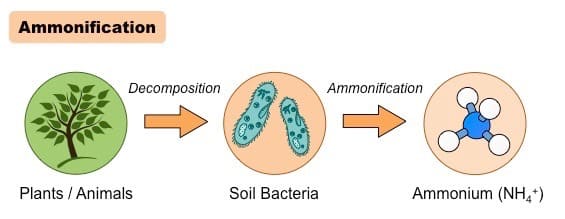
Ammonification vs. Other Processes In The Nitrogen Cycle
| Process | Description | Key Organisms |
|---|---|---|
| Nitrogen Fixation | Conversion of atmospheric nitrogen (N2) into ammonia (NH3) by nitrogen-fixing bacteria | Rhizobium, Azotobacter |
| Nitrification | Conversion of ammonia into nitrites (NO2-) and then into nitrates (NO3-) by nitrifying bacteria | Nitrosomonas, Nitrobacter |
| Ammonification | Decomposition of organic matter into ammonia by decomposers | Decomposer bacteria, fungi |
| Denitrification | Conversion of nitrates back into atmospheric nitrogen (N2) | Denitrifying bacteria |
Tips, Tricks, And Strategies For Studying Ammonification
- Draw Diagrams: A diagram showing the nitrogen cycle with an explicit representation of ammonification and what it entails makes the idea easier to understand.
- Mnemonics: To remember the steps of the nitrogen cycle, write down or teach: "FANAD" (Fixation, Ammonification, Nitrification, Assimilation, Denitrification).
- Multimedia Learning: See animations or videos to understand the significance of ammonification in the context of the nitrogen cycle.
- Real-Life Connection: Compare ammonification to other processes that people commonly use in composting dead organic matter to produce nutrients for plants.
Weightage Of Questions On Ammonification In Exams
| Exam Type | Weightage of Ammonification |
|---|---|
| CBSE Board Exams | 4-6% |
| NEET | 1-2% |
| Nursing Entrance Exams | 2-3% |
| Paramedical Entrance Exams | 1-2% |
Types Of Questions Asked On Ammonification In Exams
| Exam Type | Types of Questions |
|---|---|
| CBSE Board Exams | Definitions, role in the nitrogen cycle, examples |
| NEET | MCQs, assertion-reason questions, reaction-based questions |
| Nursing Entrance Exams | True/false, scenario-based questions related to soil health |
| Paramedical Entrance Exams | Case studies on soil nitrogen management |
Also Read:
Recommended Video On Ammonification
Frequently Asked Questions (FAQs)
Ammonification Definition:Ammonification is the process of converting organic nitrogen from dead organisms to ammonia by microorganisms.
Ammonification Importance
It provides essential nitrogen for plants that do not have a symbiotic relationship.
It maintains the ecosystem by recycling the dead organisms organic nitrogen compounds such as amino acids,dna,urea.
It balances the nitrogen in the atmosphere and the ecosystem .
It is crucial as it converts organic nitrogen into inorganic nitrogen ammonia.
Nitrogen is the abundant gas in the atmosphere which makes78% of air.It is essential component for proteins,dna.It is required for growth,metabolic process and reproduction.Nitrogen is converted to ammonia and nitrates by nitrifying and ammonifying bacteria.So that plants can access it.
Ammonification happens when a plant or animal dies or excretes wastes such as urea,feces.these dead organisms and wastes contains nitrogen compounds such as amino acids.Ammonifying bacteria converts these organic nitrogen from dead organisms and wastes into inorganic ammonia.Ammonia combines with hydrogen and forms ammonium that goes to next step of cycle.
ammonification-It is the process of conversion of organic nitrogen(dead organisms,feces,urea) into ammonia by bacteria or fungi.they break down dead organisms.
Nitrification-Oxidation of ammonium ions into nitrate by nitrifying bacteria.nitrate is assimilated by plants.
Symbiotic bacteria(depending on other organisms) are found at the root nodules of plants and they take shelter from plants by providing nitrogen to plants through conversions.
Non symbiotic bacteria(not dependent on other organisms) live freely and they fix nitrogen for their own purpose.
Ammonification is the step of the nitrogen cycle in which organic nitrogen from decaying organisms is converted into ammonia or ammonium ions that might then be absorbed by plants or otherwise processed into nitrates.
Ammonification is the process in which organic nitrogen compounds are converted into ammonia (NH₃) or ammonium ions (NH₄⁺) by bacteria and fungi.
Also Read
30 Sep'24 09:24 AM
18 Sep'24 06:01 PM
18 Sep'24 03:37 PM
26 Aug'24 03:39 PM
26 Aug'24 03:32 PM
26 Aug'24 03:31 PM
26 Aug'24 03:27 PM
26 Aug'24 03:22 PM
26 Aug'24 03:10 PM
26 Aug'24 03:03 PM

