Biomolecules: Chemical Composition Analysis
Biomolecules are complex organic compounds like carbohydrates, proteins, lipids, and nucleic acids that form the basis of all living organisms. They enable vital biological functions, including metabolism, genetic transmission, and energy production. Essential for NEET and Class 11 Biology under Biomolecules.
This Story also Contains
- What are Biomolecules?
- Types of Biomolecules
- Analysis of Chemical Composition
- Carbohydrates: Structure and Functions
- Proteins: Structure and Functions
- Lipids: Structure and Functions
- Nucleic Acids: Structure and Functions
- Methods of Chemical Composition Analysis
- Applications of Biomolecule Analysis
- Biomolecules NEET MCQs (With Answers & Explanations)
- Recommended for Biomolecules: Chemical Composition Analysis
What are Biomolecules?
Biomolecules are organic macromolecules that play vital roles in the existence of living organisms. These include carbohydrates, proteins, lipids and nucleic acids. Biomolecules are involved in all the structures and activities that occur in cells since they are the fundamental units of life. The knowledge of biomolecules is essential in biology as they are involved in all cellular biochemical activities ranging from energy production to DNA synthesis and transmission.
Types of Biomolecules
The various types of biomolecules are:
Carbohydrates
Carbohydrates are the most abundant biomolecules on Earth. They are organic molecules composed of carbon, hydrogen, and oxygen. They can be and often are simple sugars, but can also be complex polysaccharides.
Proteins
Proteins are large biomolecules that are created from amino acids that are joined by peptide bonds to form structures with great complexity and intricacy regarding their shape.
Amino acids are the building blocks of proteins. Protein is also known as an amino acid polymer because it is made up of organic compounds which are amino acids, and 20 different types of amino acids play a role in the protein variant.
Lipids
Lipids include compounds soluble in nonpolar solvents. They contain a large number of carbon-hydrogen atoms in long chains or the form of rings.
Examples of lipids include the usual lipids, fats and oils (triglycerides), phospholipids that set up cell membranes, and sterol–cholesterol.
Nucleic Acids
Nucleic acids can be defined as large biomolecules that are made up of a chain of nucleotides. A nucleotide is a molecule that has both a sugar as well as a phosphate group and contains a nitrogenous base.
Deoxyribonucleic acid or DNA is an example of a genetic molecule that contains information and ribonucleic acid or RNA is responsible for the expression of the stored information.
Analysis of Chemical Composition
The chemical composition is discussed below:
Basic Elements (C, H, O, N, P, S)
The basic elements form the basis of carbohydrates, proteins, lipids, and nucleic acids.
Elements | Features |
Carbon |
|
Hydrogen |
|
Oxygen |
|
Nitrogen |
|
Phosphorus |
|
Sulfur |
|
Types of Bonds (Covalent, Ionic, Hydrogen, Van der Waals)
Biological molecules are held together by different types of chemical bonds, which determine their structure and stability.
Types of Bonds | Features |
Covalent Bonds |
|
Ionic Bonds |
|
Hydrogen Bonds |
|
Van der Waals Forces |
|
Carbohydrates: Structure and Functions
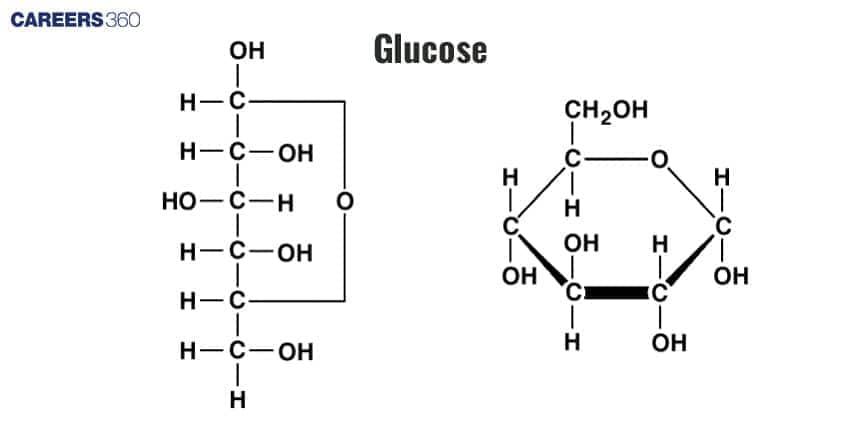
Carbohydrates are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. Many carbohydrates have the empirical formula (CH2O)n. Some also contain nitrogen, phosphorus, or sulfur. There are three major size classes of carbohydrates: monosaccharides, oligosaccharides, and polysaccharides
Monosaccharides, Disaccharides, Polysaccharides
The simplest carbohydrates are the monosaccharides, which are sugars that are made of one monomer, they include glucose.
A disaccharide is a compound that is formed when two monosaccharides join together, for instance sucrose and lactose.
Polysaccharides are large carbohydrate compounds that are characterized by long chains of monosaccharide units, examples include starch, glycogen, and cellulose.
Functions: Certain carbohydrates (sugar and starch) are a dietary staple, carbohydrate polymers (also called glycans) serve as structural and protective elements in the cell walls of bacteria, plants and in the connective tissues of animals. Other carbohydrate polymers lubricate skeletal joints and participate in cell-cell recognition and adhesion.
Chemical Test – Benedict’s Test
This test determines reducing sugars. The presence of reducing sugars can be deduced from a change of color from blue, green, and yellow to red when a carbohydrate solution is heated with Benedict’s reagent.
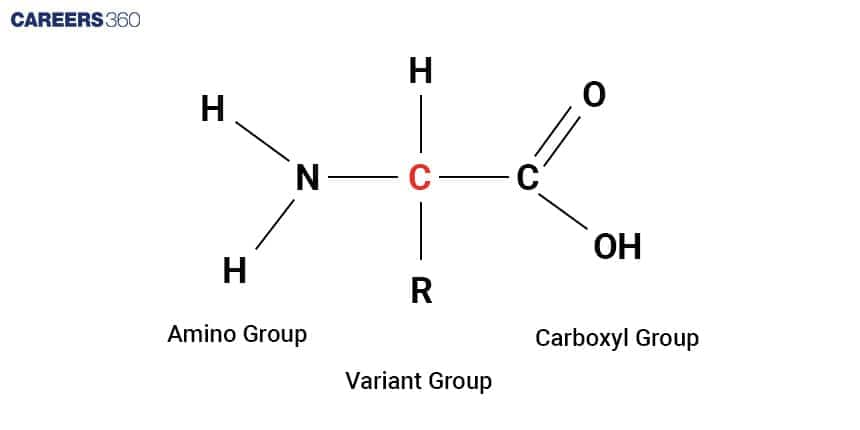
Proteins: Structure and Functions
Proteins are made from amino acids. This is polymerized through elimination synthesis or dehydration synthesis. The individual building block of each protein is called an amino acid and it has an amino group, a carboxyl group, and an amino acid specific side chain or branch (R group).
Levels of Protein Structure
There are four levels of protein structure:
Primary: The specific linear array of amino groups into a polypeptide chain.
Secondary: The secondary structure of the polypeptide chain in which the segments of the chain may coil into αhelices and βpleated sheets.
Tertiary: The gross conformation of a single polypeptide chain in which the polypeptide backbone is maintained in three dimensional shape through interactions of the side chains.
Quaternary: A condition in which many polypeptide subunits make up a protein complex.
Functions: Protein is involved in enzymatic activities, as hormones, in the structure and shape of the cells, and acts in immune defence too. Some of the examples include hemoglobin (transportation of oxygen), keratin (structural protein present in hair and nails) and insulin (hormone that helps regulate blood sugar).
Chemical Tests – Biuret Test
The Biuret test works by using a Biuret reagent that when reacts with proteins produces a violet coloration and this is due to the presence of peptide bonds.
Lipids: Structure and Functions
The common and defining feature of lipids is their insolubility in water. The biological functions of the lipids are as diverse as their chemistry.
The analysis is discussed below:
Types of Lipids (Fats, Oils, Phospholipids, Steroids)
Fats: Semisolid at room temperature and including mainly saturated fatty acids in fats.
Oils: At congealed state, oily liquid with no solid particles at room temperature and predominantly contains unsaturated fatty acids.
Phospholipids: Glycerol, two fatty acids, and phosphate groups that directly compose membranes, and are principal parts of the lipids layer.
Steroids: Derivatives of fatty acids with several rings, for example, cholesterol and steroid hormones, testosterone, and estrogen.
Functions: Fats and oils are the stored forms of energy in many organisms. Phospholipids and sterols are major elements of biological membranes. Other lipids are present in relatively small quantities, play crucial roles as enzyme cofactors, electron carriers, light-absorbing pigments, hydrophobic anchors for proteins, emulsifying agents in the digestive tract, hormones, and intracellular messengers.
Chemical Test – Emulsion Test
During the emulsion test, lipids form a cloudy white solution when ethanol and water are added to the mixture.
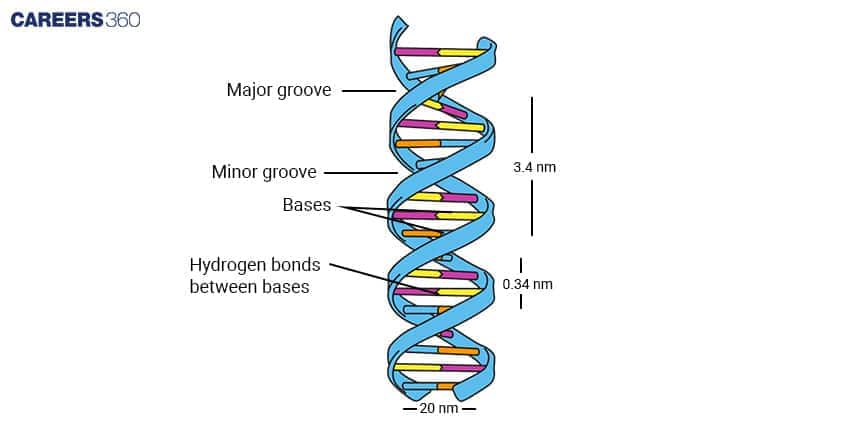
Nucleic Acids: Structure and Functions
The analysis is discussed below:
DNA and RNA
DNA is a double helix and contains the information for making proteins and RNA, which is a single chain and assists in protein synthesis and gene control.
Nucleotide Composition
Nucleotides are molecules that are made up of a sugar, a phosphate group and a nitrogenous base. They connect each other which forms the structure of DNA and RNA nucleotides.
Functions: The genetic information within DNA is expressed as genetic code within its sequence of bases. This code is then transcribed into RNA. DNA communicates this information to RNA and converts these instructions into proteins, which perform cellular operations.
Methods of Chemical Composition Analysis
The methods are discussed below:
Chromatography
This technique relies on the principle of differential migration of the components of the mixture on a stationary phase. There’s no doubt that it is used to help detect and estimate the amounts of specific compounds in multicomponent systems.
Spectroscopy
Spectroscopy is a field of study that has to do with the use of light to interact with matter to analyze the makeup of a given substance. Molecular spectroscopy such as UVVis, IR, and NMR are widely used and valuable methods in chemical analysis.
Electrophoresis
This technique employs an electric field to separate molecules according to their size and or electrical charge. It is utilized in the examination of nucleic acids as well as proteins.
X-ray Crystallography
It is a technique for finding out the atomic and molecular constitution of a crystal by analyzing the diffraction of X-rays. It is important when it comes to establishing the finer forms of biomolecules.
Use of Enzymes in Analysis
Enzymes are applied as selective reagents in detecting or measuring substances in biochemical analysis. They are widely used in diagnosis and biochemical activities.
Applications of Biomolecule Analysis
The applications are discussed below:
Medical Diagnostics
Bioanalysis of biomolecules has a critical role in the diagnosis of diseases. The prognosis of health status and the creation of molecular medicine are based on biomarkers.
Forensic Science
Analyzing biomolecules including DNA and proteins, assists in cases of human identification including criminals, forensic cases, and identification of family relationships besides identifying unknown individuals.
Biotechnology
Biotechnology involves the evaluation of biomolecules in genetic manipulation, pharmaceutical processes, and the production of industrial enzymes that improve other related sciences.
Agricultural
Knowledge of biomolecules contributes to creating GM crops, improving plants' immunity to pests and diseases and increasing the nutritional table.
Biomolecules NEET MCQs (With Answers & Explanations)
Important topics for NEET exam are:
Types of Biomolecules (Structure, Function)
Chemical Test for each Biomolecule
Practice Questions for NEET
Q1. Which of the following is present in the maximum quantity in the cellular mass?
Proteins
Amino acids
Water
All
Correct answer: 3) Water
Explanation:
The major component of cellular mass is water, which constitutes about 70-90% of the cell. It plays a vital role in biochemical reactions, transport of substances, temperature regulation, and maintaining cell structure.
Hence, the correct answer is option 3) Water
Q2. Which of the following is used for the chemical analysis of the living tissue?
Tribromoacetic acid
Tri Acetoacetic acid
Trichloroacetic acid
All of these
Correct answer: 4) Trichloroacetic acid
Explanation:
Trichloroacetic acid (TCA) is used for the chemical analysis of living tissue.
Proteins are frequently precipitated from tissue samples using TCA. Proteins and other macromolecules can be separated from the other tissue components by adding TCA which facilitates tissue examination. Additionally, it is employed in lab research to separate particular substances such as proteins and nucleic acids from cells and tissues for additional analysis.
Hence, the correct answer is option 3) Trichloroacetic acid.
Q3. While examining an organism for the presence of organic materials, micromolecules are found in
acid-insoluble fraction
acid-soluble pool
Both a and b
None
Correct answer: 2) Acid-soluble pool
Explanation:
Living tissue chemical composition is analyzed using two major methods: chemical analysis of the tissue specimen involves grinding this with trichloroacetic acid into a slurry that is later strained to get two fractions which are the filtrate, in which there occur the acid-soluble pool containing mainly macromolecules like amino acids and sugars in molecular weight lower than 1000 Da while the retentate, often called the acid-insoluble fraction, contained macromolecules like proteins, nucleic acids, and mostly insoluble lipids.
Hence, the correct answer is option 2) acid-soluble pool.
Also Read:
Recommended for Biomolecules: Chemical Composition Analysis
Frequently Asked Questions (FAQs)
The methods used to analyze the chemical composition of biomolecules are chromatography, spectroscopy, electrophoresis, Xray Crystallography and use of enzymes.
Carbohydrates supply energy and are included in the structure of cells. Proteins act as enzymes, hormones, antibodies, and structural components. Lipids are used as energy storage, formation of cell membranes, and work as signaling compounds. DNA carries hereditary information, and RNA interprets this information into proteins.
The fundamental chemical units in biomolecules are carbon hydrogens, oxygen, nitrogen, phosphorus, and sulfur. These elements combine in various ways to form the four major types of biomolecules: oval items and there are carbohydrates, proteins, lipids, and nucleic acids each with a different fourierization pattern.
Carbohydrates: Consisting of monosaccharides (single sugars), they are used to release energy and as cell support. Examples of these include glucose and cellulose.
Protein: Made of amino acids connected by peptide bonds they function as enzymes, collagen, or even hormones anotherser.
Lipid: Contain fatty and glycerol, they are the sources of energy, constitute cell walls in the form of phospholipids, and act as signal instances in the form of steroids.
Nucleic acid: DNA and RNA are examples of such complex molecules; DNA contains stored blueprints for constructing different entities in an organism while RNA is involved in constructing proteins.
Biomolecules are molecules that are vital in biological processes, these are carbohydrates, proteins, lipids, and nucleic acids. They are significant because they make the construction of control chechemicalactions, contain genetic data, and serve many other needs that are essential for the genesis, evolution, and sustenance of life forms.

