Lysosomes: Definition, Types, Examples, Diagram, Function
Lysosomes are membrane-bound organelles packed with hydrolytic enzymes that break down cellular waste, worn-out organelles, and invading pathogens. Often called the “suicidal bags” of the cell, they play vital roles in autophagy, apoptosis, and immune defense, making them a crucial concept in NEET and Class 11 Biology.
This Story also Contains
- What are Lysosomes?
- Historical Background (Discovery by Christian de Duve)
- Structure of Lysosomes
- Why are Lysosomes known as Suicidal Bags?
- Types of Lysosomes
- Function of Lysosomes
- Lysosomal Enzymes – Formation and Role
- Lysosomal Storage Disorders (LSDs)
- Lysosome in Plant Cell vs Animal Cells
- Biogenesis and Maintenance of Lysosomes
- Applications and Relevance
- Lysosome NEET MCQs (With Answers & Explanations)
- Recommended Video for Lysosome
What are Lysosomes?
Lysosomes are single membrane-bound organelle localised in eukaryotic, acclimated as the garbage disposal system of the cell. They include different hydrolytic enzymes that are capable of degrading macromolecules like protein, lipids, nucleic acids, and carbohydrates into smaller and simpler units. Lysosomes are necessary for the digestion and the destructive and recycling processes of cells.
Historical Background (Discovery by Christian de Duve)
Lysosomes have been identified since the mid-1950s by a Belgian cytologist by the name of Christian de Duve. De Duve spotted membrane-bound vesicles holding strong hydrolytic enzymes in cells, he called them lysosomes. He made a discovery that changed the principle of cellular biology and explained how a cell gets its nutrients and disposes of waste products.
Structure of Lysosomes
The structure of the lysosome is described below-
Membrane and Lipid Bilayer
Lysosomes are single membrane-bound organelles that are bounded by a lipid bilayer. The lysosome has different proteins in its membrane pore that are responsible for importing macromolecules headed to the lysosome.
Hydrolytic Enzymes & Acidic Environment
The lysosome contains hydrolytic enzymes which comprise proteases, lipases, nucleases, and glycosidases. These enzymes are optimally active in the acidic pH of about 4.5 to 5.0. The acidic pH is facilitated by a proton pump in the lysosomal membrane that translocates hydrogen ions (protons) into the lysosome. Thus creating an environment that favours enzyme function.
Why are Lysosomes known as Suicidal Bags?
Lysosomes are popularly called suicidal bags as they perform self-degradation. This occurs under various circumstances, especially when the lysosomal membranes are disrupted and thus release the hydrolytic enzymes into the cytoplasm. As such, they can lyse the cell and break down the cellular material leading to death.
This process is paramount in situations where cellular stress or injury occurs. This results in the rupture of the lysosome leading to cell damage and ultimately death. Therefore, the nickname “suicidal bags” refers to the dangerous destructive capability of lysosomes if their membranes are interrupted.
Types of Lysosomes
The different lysosomal types are discussed below-
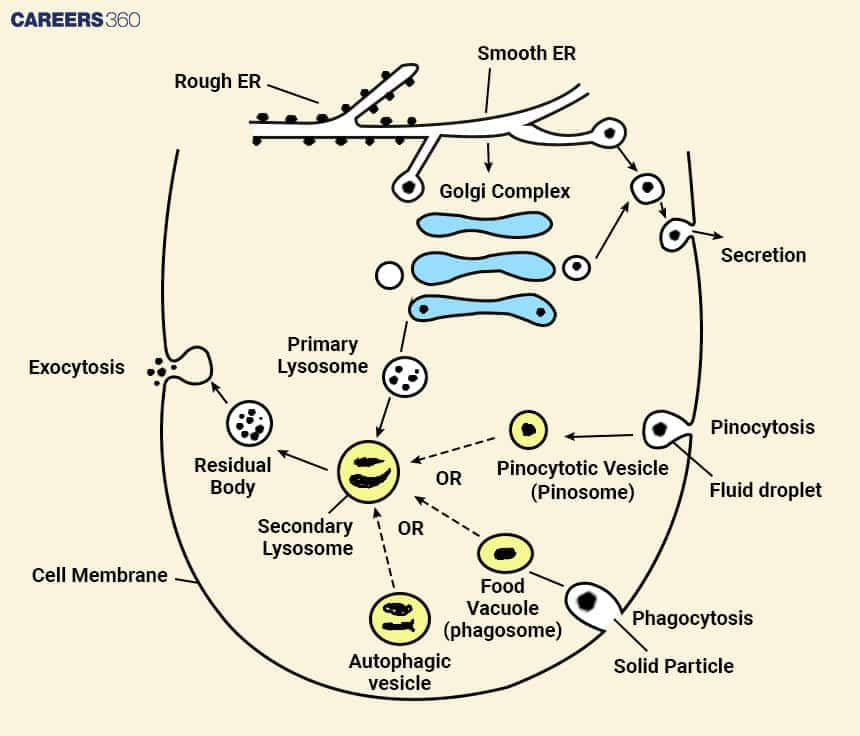
Primary lysosomes
The primary lysosomes are the new-forming lysosomes, which contain inactive hydrolytic enzymes. These enzymes are biosynthesized in the endoplasmic reticulum and then transported to the Golgi apparatus where they are modified before being shipped to the lysosomes.
Secondary Lysosomes
Some of the structures that arise from the fusion of primary lysosomes with other vesicles are known as secondary lysosomes. On fusion, the hydrolytic enzymes in the primary lysosomes get activated, break down the engulfed materials and discharge their elements for re-cycling or other uses.
Autophagosomes & Autolysosomes
Autophagosomes are the vesicles that are formed during autophagy, cellular components like damaged organelles or protein aggregates are enveloped within the double membrane structure. These autophagosomes later fuse with the lysosomes to form the so-called autolysosomes where the engulfed material is degraded by the enzymes of lysosomes.
Endolysosomes
Endolysosomes are vesicles derived from the fusion of endosomes with lysosomes. These are small spherical structures surrounded by a membrane and play a part in sorting and transporting materials. Endosomes that transport the internalized material fuse with lysosomes to form endolysosomes through which the endocytosed materials are degraded by lysosomal enzymes.
Residual Bodies
These are the lysosomes with undigested food material. They fuse with the plasma membrane and remove the debris through exocytosis.
Function of Lysosomes
Following describe the functional aspect of lysosomes-
Intracellular Digestion
Lysosomes act as the sewerage system of the cell with the function to degrade all types of macromolecules like proteins, lipids, nucleic acids, and carbohydrates into their basic components.
Lysosomes also act as garbage disposal where damaged organelles, cell debris and other unwanted materials get degraded. It aids the cell in recycling nutrients and components for use in the construction of new cellular structures.
Autophagy (Self-Digestion)
Autophagy is a process in which cellular components include organelles and proteins, which are no longer functional to be exchanged by a double membrane called autophagosomes. These autophagosomes then fuse with lysosomes and form what are referred to as autolysosomes within which the enclosed materials are then broken down by lysosomal enzymes. This process is crucial for the sustenance of a stable environment within the cell and for avoiding the buildup of hazardous by-products of cellular metabolic activity.
Pathogen Destruction & Immunity
Lysosomes help the cell in its defence mechanisms as they digest antibodies and any other foreign material like bacteria and viruses taken in by the cell. Cells including macrophages and neutrophils recognize pathogens and engulf them into the phagosomes that later on fuse with lysosomes thus forming phagolysosomes where the pathogens are destroyed.
Proteases and nucleases that are present in lysosomes break down the proteins and nucleic acids of the pathogens. This process is important in the protection of the cell against microorganisms’ intrusions and general immune system response.
Apoptosis (Programmed Cell Death)
Lysosomes actively participate in apoptosis also known as programmed cell death through the release of hydrolytic enzymes into the cytoplasm of the cell and hence in degrading almost all cellular organelles culminating in the death of the cell.
Lysosome-dependent apoptosis is crucial in the process of embryonic development, tissue remodelling, and the clearance of unhealthy, damaged, or infected cells. Dysregulation of lysosomal-dependent apoptosis leads to diseases such as cancer, neurodegenerative diseases, and autoimmune diseases.
Lysosomal Enzymes – Formation and Role
Lysosomal enzymes are synthesized mainly in the ER. Once synthesized, these enzymes are translocated to the Golgi apparatus, organelle where the enzymes are processed and matured further. Within the Golgi apparatus, the lysosomal enzymes undergo several chemical changes, one of which is glycosylation which involves the addition of specific carbohydrate residues.
Lysosomal Storage Disorders (LSDs)
Lysosomal storage disorders are a group of genetic diseases that are caused by the reduced activity of lysosomal enzymes; thus, the substrates fail to degrade within the lysosomes. Some of the LSDs are: Gaucher, Tay-Sachs, Pompe.
Disease | Enzyme Deficiency | Symptoms | Treatment Options |
Gaucher's Disease | Glucocerebrosidase | Enlarged liver and spleen, bone pain, fatigue, anaemia | Enzyme replacement therapy (ERT), substrate reduction therapy (SRT) |
Tay-Sachs Disease | Hexosaminidase A | Progressive neurodegeneration, muscle weakness, seizures | Supportive care, experimental gene therapy |
Pompe Disease | Acid alpha-glucosidase | Muscle weakness, respiratory issues, enlarged heart | Enzyme replacement therapy (ERT), gene therapy |
Lysosome in Plant Cell vs Animal Cells
In plant cells, the structures functionally related to lysosomes are termed as the lytic vacuoles or simply vacuoles. Whereas in the animal cells lysosomes are strictly involved in cellular digestion and waste disposal, vacuoles in the plant cells have many other functions.
Phagosomes of plant cells can be compared to the lysosomes in animal cells as they are involved in the process of intracellular digestion and are equipped with hydrolytic enzymes that degrade macromolecules. They are involved in cellular waste disposal since they break and recycle other cellular structures and products.
Biogenesis and Maintenance of Lysosomes
The process of biogenesis and maintenance of lysosomes is described below:
Golgi Apparatus & M6P Tagging
Lysosomes are obtained through the Golgi apparatus and endosomes. The newly formed lysosomal enzymes have to be retrogradely transported with the help of mannose-6-phosphate (M6P) receptors in the Golgi apparatus. These enzymes are then incorporated within structures known as primary lysosomes, which in turn pinch off from the Golgi apparatus.
Primary lysosomes combine with endosomes thereby forming the secondary lysosomes or endosome-lysosomes. Endosomes host materials that have been brought in from the cell surface and when these two come together with the primary lysosome then we have substrates for digestion in lysosomes.
Role of mannose-6-phosphate (M6P) tagging: For the targeting of lysosomal enzymes to the lysosomes, the process of mannose-6-phosphate (M6P) tagging is very important. In the Golgi complex, the lysosomal enzymes are modified with the M6P group which helps in targeting and shuttling the enzymes to lysosomes.
Regulation via TFEB
Role of transcription factors: User-defined transcription factors such as transcription factor EB (TFEB) are critical in controlling the process of the formation of lysosomes and even their functionality. TFEB targets the genes that are involved in the formation of the lysosome, the autophagy process as well as the activity of lysosomal enzymes.
Regulation: There are various ways through which the lysosomes operate effectively and the integrity of its membrane is preserved. The lysosomal enzymes are active only within the lysosomes since they are activated at the acidic pH within lysosomes where they degrade the macromolecules.
Applications and Relevance
The application and relevance of lysosomes is described below:
Disease Treatment
Lysosomes are used in the diagnosis and treatment of various diseases, especially LSDs. Studying the lysosomal function and the dysfunctions has given an understanding of LSDs and the attempt to control them through ERT and gene therapy.
Drug Delivery
Lysosomes are employed in drug delivery vehicles to increase the efficiency of delivery of the molecules to the target cells. Liposomes and nanoparticles can also be designed to release the drug in the lysosomal matrix, which has a low pH and enzymatic environment.
Role in Cell Biology Research
Lysosomes are integral components of cellular and molecular biology. Knowledge of the role of lysosomes and their pathophysiology is crucial to the students and investigators focusing on cell biology and metabolism and degradation pathways as well as for those interested in the mechanisms of lysosomal diseases.
Lysosome NEET MCQs (With Answers & Explanations)
Important topics for NEET exams:
Structure of Lysosomes
Types of Lysosomes
Practice Questions for NEET
Q1. What will happen to a cell if the lysosome bursts?
No effect on cells.
Cell will die
Cell will survive
Reduced cell functioning
Correct answer: 2) Cell will die
Explanation:
Basically, if the lysosomes within the cell rupture, the acidic enzymes that are trapped therein will be unleashed and will severely damage the various parts of the cell, ultimately killing it.
Option (A) is incorrect because "lysosomal cell death" is caused by the permeabilization of the lysosomal membrane and the subsequent leaking of the lysosomal content into the cytoplasm.
Option (C) is incorrect because there is no chance of cell survival after the lysosomal burst.
Option (D) is incorrect because cell functioning will stop as lysosomal enzymes degrade the essential components of cells that are useful for cell functioning. Hence cell functioning will be stopped and not reduced.
Hence, the correct answer is option 2)Cell will die
Q2. Which of the following organisms will have lysosomes in its cell?
Mango tree
Sparrow
Wheat plant
Carrot grass
Correct answer: 2) Sparrow
Explanation:
Lysosomes are single membrane-bound organelles mainly found in animal cells. They serve as the digestive and recycling facility of the cell. In 1955, Christian de Duve discovered them, containing hydrolytic enzymes that function optimally at an acidic pH to break down a variety of biomolecules. They are also called "suicidal bags" because they can digest worn-out organelles and foreign substances. In contrast, lysosomes are absent in plant cells, indicating that their role is specific to the metabolism of animal cells.
Hence, the correct answer is option 2) Sparrow.
Q3. Which of the following combinations is correct for the enzymes of lysosomes?
Oxidative, active at acidic pH
Hydrolytic, active at basic pH
Synthetic, active at neutral pH
Hydrolytic, active at acidic pH
Correct answer: 4) Hydrolytic, active at acidic pH
Explanation:
Lysosomes are small, membrane-bound organelles that contain hydrolytic enzymes responsible for the breakdown of food, foreign particles, and the remnants of dead or damaged cells. These enzymes are inactivated inside the lysosomes so that they do not digest the cell itself. It is only when the substrate has reached the acidic pH of 4-5 within the lysosome that the enzymes are activated to efficiently digest the substrates to keep the cells healthy.
Hence the correct answer is Option (4) hydrolytic, active at acidic pH.
Also read:
Recommended Video for Lysosome
Frequently Asked Questions (FAQs)
These organelles are involved in intracellular digestion such as cellular debris, injured organelles, and bacteria that may have been engulfed. Further, lysosomes are involved recycling cellular components, repair of the plasma membrane, and certain signaling pathways.
Lysosomes were discovered by Christian De Duve in the 1950s. He found hydrolytic enzymes in the cells and labelled them as lysosomes. Lysosomes are present in all eukaryotic cells.
Lysosomes are also known as ’ suicidal bags ’ since they can autolyze under certain circumstances. If the lysosomal membrane is ruptured, the hydrolytic enzymes present with the lysosomes can be released which in turn digest the cell structures and cause cell death.
Some plant cells have organelles called vacuoles, which function like the lysosomes in animal cells. Vacuoles were seen to have intracellular digestion and waste disposal activities besides being involved in nutrient storage, turgidity pressure, and detoxification.
This machinery is exclusive to lysosomes and other organelles and the proteins needed by lysosomes are synthesized in the endoplasmic reticulum or ER which is the membrane network in the cell responsible for protein synthesis. These enzymes are synthesized in the ER and following that, pass through the Golgi for further modification.
