Oxygen Dissociation Curve: Definition, Function And Examples
What Is the Oxygen Dissociation Curve?
The oxygen dissociation curve is central to describing how oxygen is associated with and discharged from haemoglobin in the blood. The theory related to this curve goes on to explain how, effectively, oxygen is supplied to tissues in our body and how carbon dioxide is removed.
Basics
Heme is a protein in the blood cells that carries oxygen from the air a person breathes to different parts of their body. The information about the relationship entered above between the partial pressure of oxygen and the percentage of haemoglobin saturated with oxygen is held within a graph.
Oxygen Binding Of Haemoglobin
Hemoglobin binds to oxygen cooperatively. The idea here is that, once an oxygen molecule has bound to the active site, this will increase its affinity for all the remaining sites to bind with the oxygen. This cooperative nature of binding with oxygen gives its characteristic sigmoid shape to the oxygen dissociation curve.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
NEET Important PYQ's Subject wise: Physics | Chemistry | Biology
New: Meet Careers360 B.Tech/NEET Experts in your City | Book your Seat now
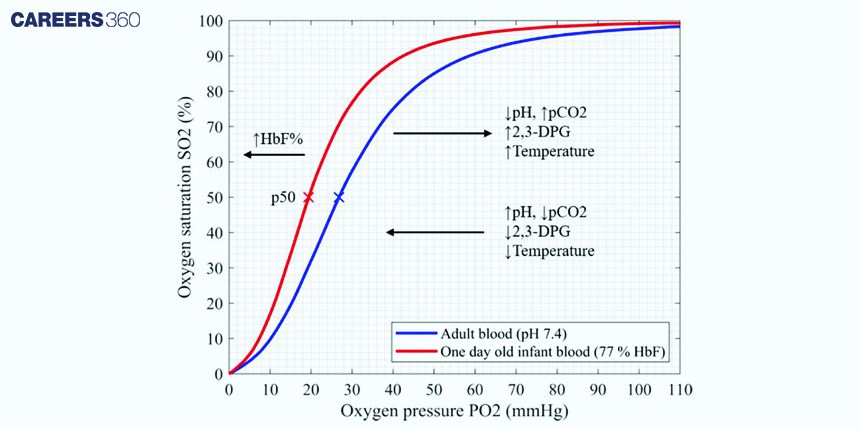
Factors Affecting The Shape Of The Oxygen Dissociation Curve:
Partial pressure of oxygen (pO2): This exerts the most marked influence on the curve. High pO2 in the lungs favours haemoglobin binding to oxygen, and values of low pO2 favour the release of oxygen in tissues.
pH: A decrease in pH: an increase in hydrogen ion concentrations—caused a decrease in affinity as P50 increased hence shifting the curves to the right; referred to as the Bohr effect.".
Carbon dioxide levels: Higher amounts of CO2 will shift the pH further right; thus, less binding with the oxygen is allowed by haemoglobin—meaning that then more oxygen is released.
Temperature: The curve shifts rightward as temperature increases. This has the effect of lowering haemoglobin's affinity for oxygen.
2,3-Bisphosphoglycerate: This is a molecule that binds with haemoglobin to decrease its affinity for oxygen. The net effect of this binding would be a shift to the right—favouring increased delivery of oxygen to tissues.
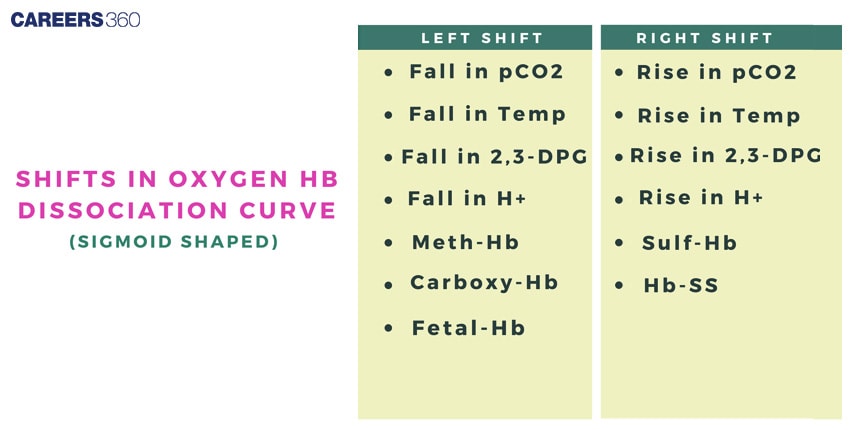
Shifts In The Oxygen Dissociation Curve
Right Shift: Less haemoglobin affinity for oxygen; hence there will be an improved potential to give off oxygen to the tissues. Increased scavenge due to increased pCO2, higher temperatures, and decreased pH and increased 2,3-BPG.
Right Shift: A decreased affinity of haemoglobin to oxygen means a further enhancement in the ability of haemoglobin to give up oxygen to the tissues. This may happen with an increased pCO2, increased temperature, decreased pH, and increased 2,3-BPG.
P50 Value
It is the value of P50, which is the partial pressure required to produce 50 per cent saturation of haemoglobin. It happens to be a significant measure related to haemoglobin affinity for oxygen. An increase in P50 denotes a rightward shift as opposed to a decrease which signifies a leftward shift.
Physiological Relevance
Oxygen loading in the Lungs: An elevated pO2 ensures optimum loading of oxygen onto the haemoglobin of the lungs.
Oxygen unloading in the tissues: Low pO2 synergizes with other tissue factors to allow the offloading of oxygen from the Hb molecule, most effectively delivering it.
Adaptations: There are shifts in the oxygen-dissociation curve, as needed, to enable physical changes to adapt efficiently to the physical change for efficient transfer of oxygen, such as at high altitudes or during exercise.
Carbon Monoxide Poisoning: Carbon monoxide binds much more firmly to haemoglobin than oxygen and therefore decreases its oxygen-carrying capacity, shifting the curve to the left.
Sickle Cell Anemia: Abnormal haemoglobin of sick cell anaemia shall direct an abnormal oxygen dissociation curve and hence affect the delivery of oxygen.
Recommended video for Oxygen Dissociation Curve
Frequently Asked Questions (FAQs)
The oxygen dissociation curve is a graph showing the relationship of partial pressure of oxygen with per cent haemoglobin saturated with oxygen.
Increased carbon dioxide, lower pH, increased temperature, and increased 2,3-BPG all produce a rightward shift. Therefore, the rightward shift gives haemoglobin a lesser affinity for oxygen.
Changes in the oxygen affinity of haemoglobin due to changes in pH and carbon dioxide generally a shift to the right in the oxygen dissociation curve.
Because there is cooperative binding between the molecules of oxygen in haemoglobin, the binding of one molecule of oxygen increases the affinity for the binding of other molecules of oxygen.
Anemia states have decreased total haemoglobin concentrations and therefore may shift the right oxyhaemoglobin dissociation curve, indicating a reduced affinity for oxygen and a reduced carrying capacity for it.
Also Read
29 Nov'24 11:00 PM
27 Nov'24 01:11 PM
26 Nov'24 11:17 AM
26 Nov'24 10:17 AM
25 Nov'24 07:51 PM
25 Nov'24 07:19 PM
25 Nov'24 11:48 AM
18 Sep'24 06:39 PM
18 Sep'24 06:35 PM
18 Sep'24 04:11 PM