Study Of Osmosis By Potato Osmometer: Diagram, Experiment
The Potato Osmometer Experiment is a classic biology demonstration of osmosis in plant cells. This experiment clearly shows osmosis using a raw potato, a sugar solution, and water. The potato osmometer acts as a natural model to explain osmosis in plant tissues. Osmosis is the movement of water molecules across a semipermeable membrane. The experiment highlights how water moves across a semipermeable membrane from high water potential to low water potential. It visualises endosmosis, exosmosis, water potential gradient and plant- water relations.
This Story also Contains
- What is a Potato Osmometer?
- Aim of the Potato Osmometer Experiment
- Theory of Osmosis in Plant Cells
- Materials Required for Potato Osmometer Experiment
- Procedure (Step-by-Step)
- Observation of Osmosis in Potato Cells
- Conclusion: Potato Osmometer Experiment Findings
- Applications in Plant Physiology
- Potato Osmometer NEET MCQs (With Answers & Explanations)
- Recommended video on the "Study Of Osmosis By Potato Osmometer"

In this potato osmometer experiment setup, sugar solution inside the potato creates a water potential gradient. Water moves from high potential to low potential gradient. The rise in the liquid level proves osmosis clearly. This potato osmometer experiment shows how water absorption in plants happens, maintains cell turgidity and the role of the semipermeable membrane in biology. Short, visible results of the potato osmometer water level rise make the principle of osmosis in plant cells easy to understand.
What is a Potato Osmometer?
A potato osmomter is a hollow cavity made in a peeled potato and filled with a concentrated sugar solution. The potato is then placed in a beaker of water, allowing water to move through the potato’s semi-permeable cell walls into the cavity by osmosis. The resulting rise in volume causes the liquid level to visually prove water movement.
This experiment helps students understand concepts such as
Osmosis in plants cells
Water potential gradient
Endosmosis and exosmosis
The role of semi-permeable membranes in the living world.
Aim of the Potato Osmometer Experiment
The experiment aims to demonstrate the process of osmosis in plant cells. It shows how water moves across a semipermeable membrane (potato cell walls) from a region of higher water potential to a region of lower water potential.
Theory of Osmosis in Plant Cells
Osmosis is the phenomenon where solvent molecules cross a semipermeable membrane from a more concentrated region to a less concentrated region. It will carry on till the fluid quantity is equal on both sides of the membrane, thus balancing the fluid quantity on either side of the membrane. In simple terms can be defined as osmosis, the diffusion of water from the high water potential region to the low water potential region.
In the Potato Osmometer Experiment, the potato’s tissues act as a semi‑permeable membrane, allowing water to move into the sugar solution cavity due to the osmotic potential difference between the solution and the surrounding water.
Role of the Semi-Permeable Membrane
Solvent: The fluid that moves through the semipermeable membrane
Solute: Dissolved particles ( e.g. sugar) that are present in the fluid.
Types of Solutions in Osmosis
The osmosis by potato osmoscope experiment is an important method of showing the effects of different solutions and understanding the concept of osmosis in biological systems. In osmosis, plasmolysis and deplasmolysis occur as potato cells lose water in hypertonic solutions and regain it in hypotonic solutions. Here is the table of different types of solutions and their effects on the potato cell:
Solution Type | Solute Concentration | Effect on Potato Cells |
|---|---|---|
Hypotonic | Low solute, high water | Cell becomes turgid (endosmosis) |
Hypertonic | High solute, low water | Cell becomes plasmolysed (exosmosis) |
Isotonic | Equal solute and solvent | No net movement |
Materials Required for Potato Osmometer Experiment
To study by demonstrating the osmosis process by potato osmometer:
Fresh large-sized potato tuber
Sucrose solution of 20% concentration
Beaker
Water
Scalpel or blade
Petri dish
Bell pin needle marked with waterproof ink

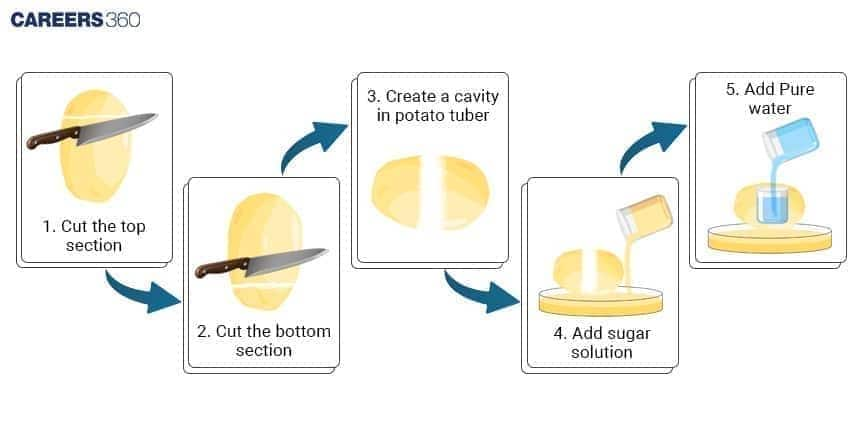
Procedure (Step-by-Step)
Cut the potato tuber into two halves using a scalpel or blade. Remove the skin and then cut these halves into square pieces.
Scoop out a small cavity from the mid-region of the potato tuber, having a minimum thickness at the base. The cavity may be square or circular.
Fill half the cavity with freshly prepared 20% sucrose solution. Insert a pin into the cavity so that its mark coincides with the level of the sucrose solution.
Place the potato osmometer in a petri dish or beaker containing water, the level is to be such that 75 % of the potato osmometer will be submerged in the water.
Allow to remain undisturbed for about 1 hour.
Observe and note the level of the sucrose solution in the osmometer at the end of the experiment.
Repeat the experiment with the cavity filled with water and the sucrose solution in the Petri dish or beaker.
Observation of Osmosis in Potato Cells
After an appropriate length of time, the sugar solution inside the potato osmometer will rise and may also become coloured. This shows water moves inside the osmometer due to endosmosis. In the potato osmometer, osmotic pressure in the middle of the process drives water into the sugar solution until equilibrium is reached.
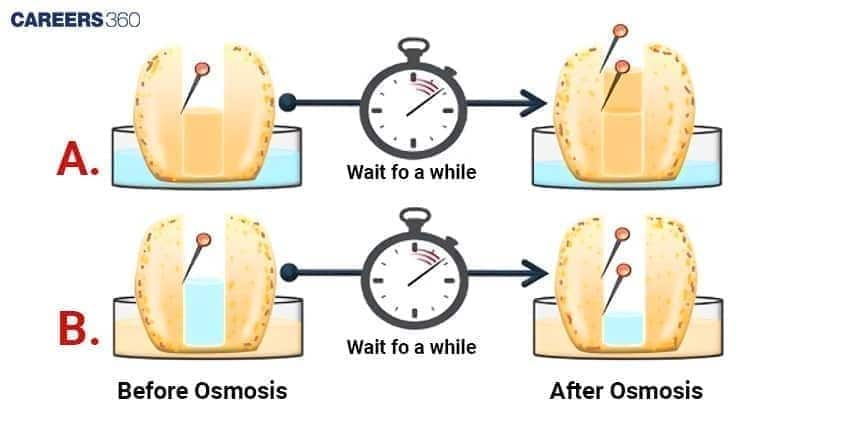
Conclusion: Potato Osmometer Experiment Findings
The conclusion of the experiment is:
In the potato osmometer experiment, there is an increase in the level of the sucrose solution because of the movement of water into the solution through endosmosis.
This process shows osmosis in plant cells, whereby the water enters the sugar solution through the tissues of the potato and acts as a selectively permeable membrane.
A water potential gradient is developed between the sucrose solution in the potato osmometer and the external water in the beaker.
The selectively permeable membrane of the potato tuber allows water to flow into the sugar solution, even though it is separated by a living potato cell.
The result of this experiment explains that it can be used as a model for the theory of osmosis, for a principle that shows how different water potential gradients can drive water movement.
Osmosis in a potato demonstrates water dynamics in a biological system, and many experiments on osmosis use this potato experiment.
Applications in Plant Physiology
The potato osmometer experiment helps to study the movement of water in plant tissues through osmosis. It helps in understanding plant-water relations and is widely used for educational demonstrations.
It helps to demonstrate the process of osmosis in plant cells.
It explains the concepts of endosmosis and exosmosis.
It shows how plants absorb water from the soil.
It is useful in teaching plant physiology in schools and colleges.
Potato Osmometer NEET MCQs (With Answers & Explanations)
The key concepts to be covered under this topic for different exams are:
Concept of Osmosis
Types of Solutions
Conclusion of the experiment
Practice Questions for NEET
Q1. Why is an osmoscope used?
To measure the osmotic pressure
To measure the solution pressure
To demonstrate the osmosis
To demonstrate the diffusion
Correct answer: 3) To demonstrate the osmosis
Explanation:
An osmoscope is a device that was designed for the demonstration as well as for the study process of osmosis. Such a device always consists of the semipermeable membrane used to separate solutions of different concentrations. When dipped in a solution, the osmoscope has the ability for water molecules in the area with a lower solute concentration to go through the barrier into the solution having a higher concentration of solutes. This activity demonstrates the theories of osmosis, especially how solvent molecules balance the distribution of solutes across a separating barrier.
Hence, the correct answer is Option (3) To demonstrate the osmosis.
Q2. Why does the level of solution rises from the initial level in the osmoscope?
Due to exosmosis of pure solvent across the potato
Due to loss of water from the potato
Due to endosmosis of pure solvent across the potato
Due to absorption of water by the potato
Correct answer: 3) Due to endosmosis of pure solvent across the potato
Explanation:
When the potato is peeled and the inner section of it is taken out with the help of a spoon or knife, it becomes U-shaped.
It is then placed in a tray having a pure solvent.
The cavity of the U-shaped potato osmoscope is filled with a 10% solution.
A pin is marked at the initial level of the 10% solution in the potato osmoscope. This pin is labelled as an initial pin.
After 2 hours, the level of the solution in the potato osmoscope will rise. This is endosmosis occurring due to the difference in the concentration of the solution.
Hence, osmoscope is a device that describes the process of osmosis.
The level of the solution in the potato osmoscope rises due to the endosmosis occurring due to the difference in the concentration of the solution.
Hence, the correct answer is Option (3) Due to the endosmosis of pure solvent across the potato.
Q3. Why do solvent particles from the pure solvent move toward the solution side in the osmometer?
Due to difference in water concentration
Due to difference in solute concentration
Both a and b
None of these
Correct answer: 1) Due to difference in water concentration
Explanation:
In an osmometer, the particles move in the direction from the pure solvent to the solution side because of concentration differences. To be more specific, osmosis involves moving water molecules to balance out the concentration of a solute on either side of a semipermeable membrane. More importantly, osmosis does not depend on the movement of the solute particles; it relies only on the gradient of water concentration molecules to achieve equilibrium.
Hence, the correct answer is Option (1) Due to the difference in water concentration
Also Read:
Recommended video on the "Study Of Osmosis By Potato Osmometer"
Frequently Asked Questions (FAQs)
A potato osmometer is a device made by scooping out a cavity in a raw potato and filling it with a concentrated solution (commonly sugar solution).
The principle of a potato osmometer is based on osmosis — the movement of water molecules across a semi-permeable membrane from a region of higher water potential to a region of lower water potential.
The potato experiment concludes that osmosis occurs in plant cells, with water moving through the semi-permeable membrane of potato tissues, demonstrating both endosmosis and exosmosis.
The osmosis potato experiment is a classic biology practical used to demonstrate the principle of osmosis—the movement of water molecules across a semi-permeable membrane from a region of higher water concentration to lower water concentration.
