Buna-S Full Form
What is the full form of Buna S?
Under its former designations of Buna S (Buna substance) and GRS(government rubber styrene), SBR(styrene-butadiene rubber) is perhaps better known. It was initially manufactured under government supervision between 1930 and 1950 and substituted for natural rubber. Styrene makes up around 23.5% of the fundamental monomers, which are butadiene and styrene. Tire manufacturing uses around a third of the world's SBR output. Seals for braking fluid applications based on non-mineral oils often employ SBR. Outside of the sealing sector, it is used today. As a result, it's mostly used for conveyor belts and car tyres. Buna materials can be used to create gaskets and O-rings alike.

The term "styrene-butadiene rubber" (SBR) refers to a group of synthetic rubbers made from styrene and butadiene (the version developed by Goodyear is called Neolite). When given additional protection, these materials exhibit strong abrasion resistance and good ageing stability. Around the world, SBR processed more than 5.4 million tons in 2012. Car tyres are produced from various types of SBR to a degree of about 50%. The qualities of the polymer are influenced by the styrene/butadiene ratio; for example, rubbers with a high styrene content are harder and less springy. Despite coming from the same monomers, SBR should not be mistaken for the thermoplastic elastomer known as a styrene-butadiene block copolymer.
History of Buna S
SBR serves as a substitute for natural rubber. Chemist Walter Bock first created it in Germany before World War II in 1929. To make Government Rubber-Styrene (GR-S) and replace the Southeast Asian source of natural rubber that was unavailable to Allied nations due to Japanese control, industrial manufacture started during World War II.
Compound Information
ASTM(American Society for Testing and Materials) D1418 Designation | SBR |
ASTM D2000/SAE J200 Type, Class | AA, AB |
Hardness (Shore A) | 40-90 |
Colour | black |
Thermal Properties
Minimum Temp. | -50°F (-45°) |
Maximum Temp. | 212°F (100°) |
Types of SBR
Styrene and butadiene, two monomers, are the sources of SBR. There are two ways to polymerize the mixture of these two monomers: in solution (S-SBR) or as an emulsion (E-SBR). E-SBR is more frequently employed.
Emulsion Polymerization
Free radicals start the process of emulsion polymerization that results in E-SBR. The two monomers, a source of free radicals, and a chain transfer agent, such as an alkyl mercaptan, are commonly added to reaction vessels. Potassium persulfate, hydroperoxides, and ferrous salts are all examples of radical initiators. Different soaps are emulsifying agents. Mercaptans, such as dodecanethiol, regulate the molecular weight and consequently the viscosity of the product by "capping" the expanding organic radicals. Usually, polymerizations are "short-stopped" when they have progressed around 70%. Different additives can be taken out of the polymer in this manner.
Solution Polymerization
An anionic polymerization method is used to create solution-SBR. Alkyl lithium compounds are what start polymerization. It is forbidden to use either water or oxygen. Given that all of the components are dissolved, the process is homogeneous, which gives the polymer's production more control and flexibility. The carbanion is created when the organolithium compound reacts with one of the monomers and adds to it, and this process continues with the subsequent monomers. S-SBR is becoming more and more popular for tyre production because it provides higher wet traction and less rolling resistance, which translates to increased safety and better fuel efficiency, respectively.
Buna-S
The product was first offered under the name Buna-S. Its name is a combination of the letters Bu for butadiene, Na for sodium (referred to as natrium in several languages, including Latin, German, and Dutch), and S for styrene. An additional copolymer is Buna-S.
Synthesis of Buna-S
It is made by copolymerizing sodium with 25% of 1,3-butadiene and 75% of styrene.
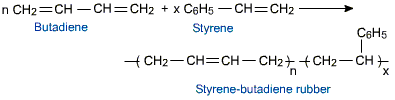
Buna-S is created when styrene interacts with butadiene, causing the double bond to dissolve and the addition of a butadiene group.
Properties
Property | S-SBR | E-SBR |
Tensile strength (MPa) | 18 | 19 |
Elongation of tear (%) | 565 | 635 |
Mooney viscosity (100°) | 48.0 | 51.5 |
Glass transition temperature(°C) | -65 | -50 |
Polydispersity | 2.1 | 4.5 |
Application
It is a substance of commodities that competes with natural rubber. Pneumatic tyres frequently make use of elastomer. Although S-SBR is becoming more common, E-SBR is still the preferred option for this application. Other applications include gaskets, chewing gum, shoe soles and heels.
Since latex (emulsion) SBR is one of the least expensive resins for bond-coloured coatings, it is widely utilized in coated papers. In place of PVA or Polyvinyl alcohol, it is also employed in building applications as a binding and sealing agent behind renders, however, it is more expensive. In the latter use, it provides greater toughness, less shrinkage, increased flexibility, and resistance to emulsification in moist environments.
SBR is frequently employed as a component in cement-based substructural (basement)waterproofing systems, where it is combined with water to create a liquid gauging solution that is used to combine powdered tanking material into a slurry. SBR adds a degree of elasticity, improves bond strength, and lessens the possibility of shrinking.
As a component for Low Damping Rubber Surrounds, it is also used by companies that make speaker drivers. Some rubber cutting boards also employ it as a component. Along with carboxymethyl cellulose, SBR serves as a binder in lithium-ion battery electrodes as a water-based substitute for materials like polyvinylidene fluoride.
Additionally, gaskets-plate heat exchangers employ styrene-butane rubber. It can be used for aqueous systems at temperatures as low as 85 °C (358 °K). For FDM 3D or Fused Deposition Modeling 3D printing, SBS Filaments are also available.
Frequently Asked Questions (FAQs)
Buna-S is a good electrical insulator.
Terylene is a fibre-like polymer. But Buna -S- rubber is an elastomer. Elastomers are one kind of polymer having elasticity.
Neoprene, Buna-S and PMMA are chain-growth polymers.
Buna-S is a synthetic rubber and nylon-6 is a synthetic fibre. All these polymers are prepared by condensation polymerization and non-biodegradable polymers.
Buna-S | Buna-N |
|
|