CaCl2 Full Form
What is the full form of CaCl2
The full form of CaCl2 is Calcium Chloride. Calcium Chloride is an inorganic salt with the chemical formula CaCl2. At room temperature, it is a white, crystalline solid that is highly water soluble and its molecular weight is 110.98 g/mol. On reacting Calcium Hydroxide with Hydrochloric Acid, a neutralisation reaction occurs and Calcium Chloride is formed.
- What is the full form of CaCl2
- History of Calcium Chloride
- Occurrence of Calcium Chloride
- Properties of Calcium Chloride
- Structure of Calcium Chloride
- Preparation of Calcium Chloride
- Uses of Calcium Chloride
- Health Hazards Of Calcium Chloride

Ca\left ( OH \right )_{2}+2HCl\rightarrow CaCl_{2}+2H_{2}O
The hydrated solid Calcium Chloride, with the general formula CaCl_{2}.nH_{2}O and n values of 0, 1, 2, 4, and 6, are frequently found in nature. For de-icing and dust control, these compounds are primarily employed. The anhydrous salt serves as a desiccant since it is hygroscopic and deliquescent.
History of Calcium Chloride
The 15th century saw the discovery of Calcium Chloride, but until the latter half of 18th century, little was known about it. Since it wasn't generated on a large scale until the ammonia-soda method for making soda ash came into operation. All of the early studies were done with laboratory-prepared samples. Prior to its applications being found, it was originally thought of as a waste product.
Occurrence of Calcium Chloride
The rare evaporite minerals like Sinjarite (dihydrate), CaCl_{2}.2H_{2}O and Antarcticite (hexahydrate), CaCl_{2}.6H_{2}O contain Calcium Chloride. The tetrahydrate Ghiaraite, CaCl_{2}.4H_{2}O is another well-known natural hydrate. Tachyhydrite (CaMg_{2}Cl_{6}.12H_{2}O), a Calcium Magnesium Chloride mineral, and Chlorocalcite (KCaCl_{3}), a Potassium Calcium Chloride mineral, are two close related but are extremely rare minerals. The same is true for Rorisite (CaClF), which is Calcium Chloride Fluoride.
Properties of Calcium Chloride
Molecular formula = CaCl_{2}
Molecular weight = 110.98 g/mol
Density of anhydrous Calcium Chloride = 2.15 g/cm3
Melting point = 1935 ℃
Boiling point = 772 ℃
Calcium Chloride has a relatively high enthalpy change of solution due to the considerable temperature increase that happens when anhydrous Calcium Chloride salt dissolves in water. This feature serves as the basis for its widest application.
Electrolysis of molten Calcium Chloride can produce calcium metal and chlorine gas.
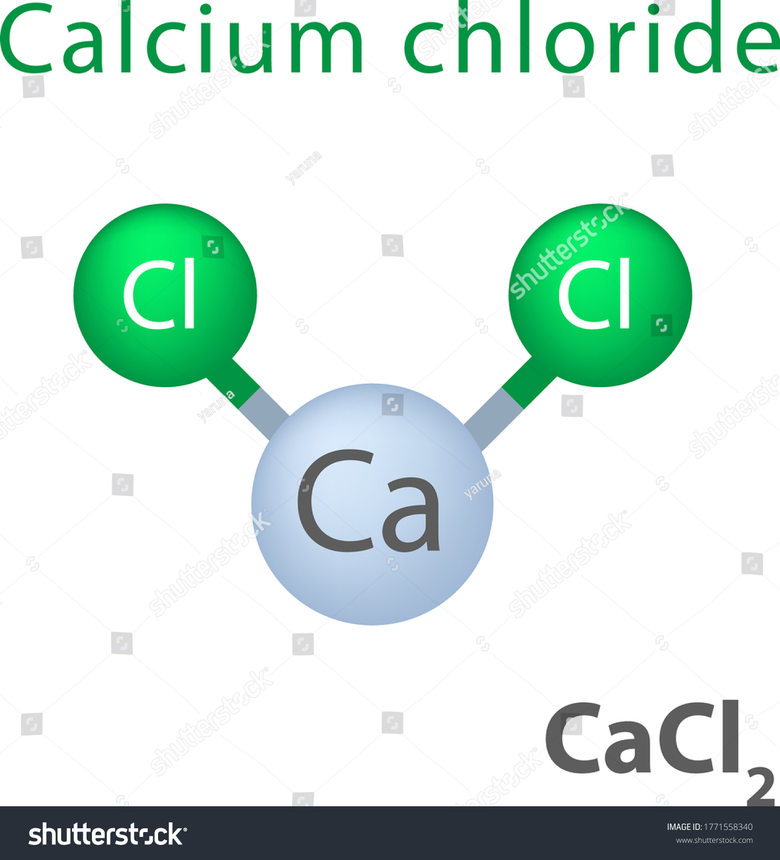
Structure of Calcium Chloride
Two ionic bonds connect the two chloride anions and the single Calcium cation in Calcium Chloride molecules. The following diagram shows the structure of the Calcium Chloride molecule. The Calcium cation has a charge of +2, while each Chloride anion has a charge of -1, and the formula is CaCl2. Therefore, the substance is electrically neutral.
Preparation of Calcium Chloride
Calcium Chloride is produced as a by-product of the Solvay process from limestone Equation is as follows:
2NaCl+CaCO_{3}\rightarrow Na_{2}CO_{3}+CaCl_{2}
Uses of Calcium Chloride
Depression of freezing point and De-icing process
Calcium Chloride is used to both prevent ice formation and de-ice by lowering the freezing point of water.
Most Calcium Chloride is used in this application.
Plants and soil are largely unharmed by Calcium Chloride. It works significantly better as a deicing agent at lower temperatures than Sodium Chloride.
It is typically distributed for this purpose in the form of prills, which are tiny, white spheres with a diameter of a few millimetres.
Additionally, it is used in commercial and residential chemical air dehumidifiers.
Road Surfacing
The second-largest use of Calcium Chloride takes advantage of both its high hygroscopicity and the tactility of its hydrates; the process of hydration of Calcium Chloride is exothermic.
A concentrated solution prevents dust from forming by maintaining a liquid layer on the surface of dirt roads.
It creates a layer of protection by keeping the smaller dust particles on the road.
Allowing these to blow away will cause the big aggregate to start moving around and eventually cause the road to collapse.
Using Calcium Chloride can cut the demand for grading and fill-in materials by up to 50% and 80%, respectively.
Food Industry
The recommended daily consumption of Calcium Chloride as a food ingredient is 160-345 mg.
Calcium Chloride is used as a firming agent in canned vegetables and when forming soybean curds into tofu.
It is frequently used as an electrolyte in bottled water, sports drinks, and other beverages.
Pickles are flavoured with Calcium Chloride, which has a very salty flavour without adding more sodium to the dish.
Chocolate bars with caramel filling can be prevented from freezing by Calcium Chloride's ability to lower freezing points.
The texture of sliced apples is frequently maintained by adding Calcium Chloride.
To maintain the natural balance between Calcium and protein in Casein during the cheesemaking process, Calcium Chloride is occasionally added to processed (pasteurised/homogenised) milk.
Breweries
Calcium Chloride is occasionally used in the brewing of beer to make up for mineral deficits in the brewing water. It can alter yeast function during fermentation as well as flavour and chemical reactions throughout the brewing process.
Laboratory operations
Calcium Chloride is routinely placed inside drying tubes. To produce sodium carbonate, kelp is dried with Calcium Chloride.
The FDA has approved anhydrous Calcium Chloride as a packaging aid to maintain dryness.
The hydrated salt can be dried for future use, but if heated quickly, it will dissolve in its own hydration water and solidify into a hard amalgam when cooled.
Other uses
Calcium Chloride is used as an ingredient in plastics, fire extinguishers, and blast furnaces to prevent scaffolding (clumping and adhesion of elements that impede the furnace charge from descending), and used as a thinner in fabric softener.
Heating pads and self-heating cans utilise calcium Calcium Chloride's exothermic dissolution.
In oil industries, the density of brines free of solids is increased by adding Calcium Chloride.
Additionally, it is employed to prevent clays in the water phase of inverted emulsion drilling fluids from swelling.
Health Hazards Of Calcium Chloride
Although the non-hydrated salt is non-toxic in small amounts when wet, its significant hygroscopic qualities pose considerable risks.
Calcium Chloride can cause irritation by drying out moist skin.
If solid Calcium Chloride is swallowed, it can burn the lips and oesophagus since it dissolves exothermically.
Consuming concentrated liquids or solid calcium chloride may irritate the stomach or result in an ulcer.
Calcium Chloride ingestion can cause hypercalcemia.
Frequently Asked Questions (FAQs)
Due to the hygroscopic nature of Calcium Chloride, on exposure to air it absorbs the moisture from the atmospheric air and forms a colourless solution.
The aqueous solution of Calcium Chloride is a good conductor of electricity and can be classified as a strong electrolyte as well.
Calcium Chloride being a hygroscopic salt acts as a good desiccant hence it has this property intact from a temperature range of -32 °C to 80°C.
Ingestion of Calcium Chloride can burn the mouth and throat and can cause dryness if it comes in contact with the skin.
The shelf life of Calcium Chloride is about 36 months hence it can be used for 36 months once opened if it is stored well in air tight bottles in moisture-free conditions.