CaCO3 Full Form
What is the full form of CaCO3?
CaCO3 is also known as calcium carbonate. It is present in many forms such as in Marbles and limestones.
It can find it in School classrooms where the use of chalk has been done. All forms of calcium carbonate are similar in chemical properties and they differ only in their physical properties. These different forms of calcium carbonate are termed calcite.
Calcium carbonate is a non-toxic substance. Calcium carbonate is a non-poisonous substance. It is usually white in color. This chemical is odorless and it naturally occurs in chalk, Marble, and limestone. It acts as an antacid or it can be used as a calcium supplement. It found its use as a filler in cosmetics and also added to swimming pools as a disinfectant agent. It is used as a pH corrector. There are many biological sources of calcium carbonates that include eggshells and snail shells. Some of the calcium carbonate sources like plankton and sponges are found in shallow water because there is abundant light and filterable food present for them.
- Structure of Calcium Carbonate
- Commercial Production of Calcium Carbonate :
- Different Types of Calcium Carbonate That Occurs in the Environment :
- Preparation of Calcium Carbonate :
- Physical Properties of Calcium Carbonate :
- Application of Calcium Carbonate :

Many dark green vegetables contain large quantities of calcium carbonate but these do not function as an industrial source of calcium carbonate. calcium carbonate acts as an antacid to treat upset stomachs and also the acid in digestion.
Structure of Calcium Carbonate
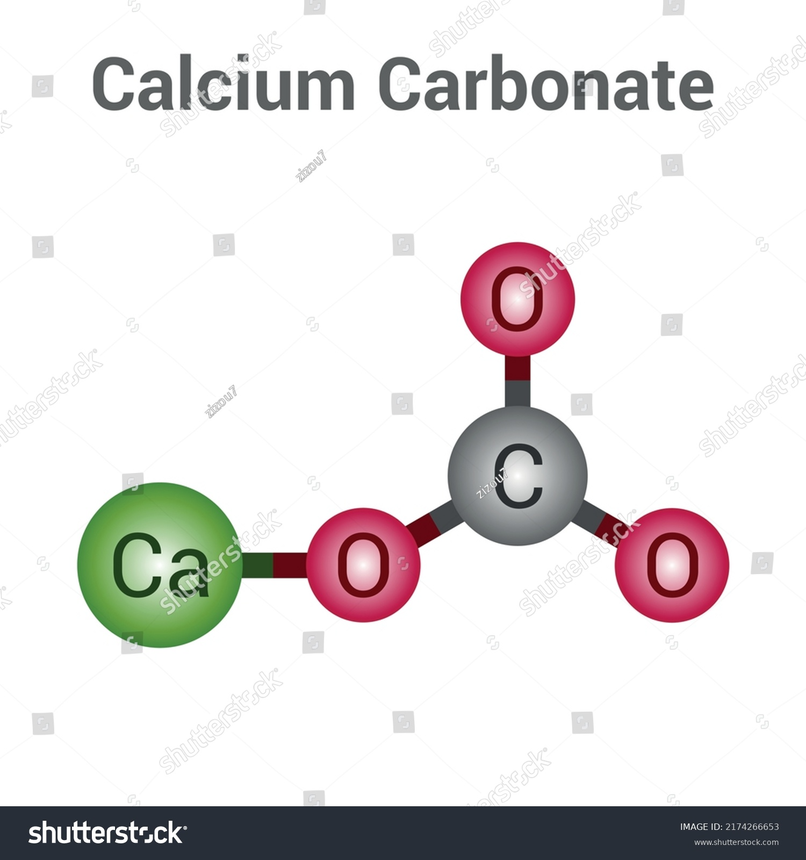
Commercial Production of Calcium Carbonate :
Calcium carbonate is commercially available in two different type
Ground calcium carbonate :
It is produced by the extraction and processing of naturally occurring deposits in the Earth's crust. It is present in the crystalline form and its shape is rhombohedral. This compound has a distribution of broader size.
Precipitated calcium carbonate :
This type of calcium carbonate is produced by the chemical precipitation of the carbocation process. This can also be produced as a byproduct of some bulk chemical processes. The shape of precipitated calcium carbonate depends on the product from which it is obtained.
Different Types of Calcium Carbonate That Occurs in the Environment :
All the forms of Calcium carbonate are very useful. There are mainly three different types of calcium carbonate that are normally present in the environment. The properties of three of them are defined below :
Calcite: This type of calcium carbonate is the most stable form. It is the least soluble in water. This type of calcium carbonate is found in sedimentary and igneous rocks. It is used in construction materials and agriculture soil treatment.
Argonite: This form is less stable than the calcite form. This type of calcium carbonate is formed by some biological processes that include precipitation from marine environments. It is used to maintain pH level and it is used to remove pollutants such as zinc.
Vaterite: This type of calcium carbonate is hexagonal in shape and it is less stable than calcite and aragonite.
It is present naturally in Spring and organic tissues.
Preparation of Calcium Carbonate :
This type of compound is prepared by using slaked lime and calcium dioxide as raw materials. When slaked lime reacts with calcium dioxide in excess amounts then it leads to the formation of calcium carbonate. The reaction usually carries in normal conditions of room temperature.
Calcium carbonate is usually created with calcium ions in hard water that react with the carbonate and produce limescale.
Another method of preparation is to react Calcium oxide with carbon dioxide
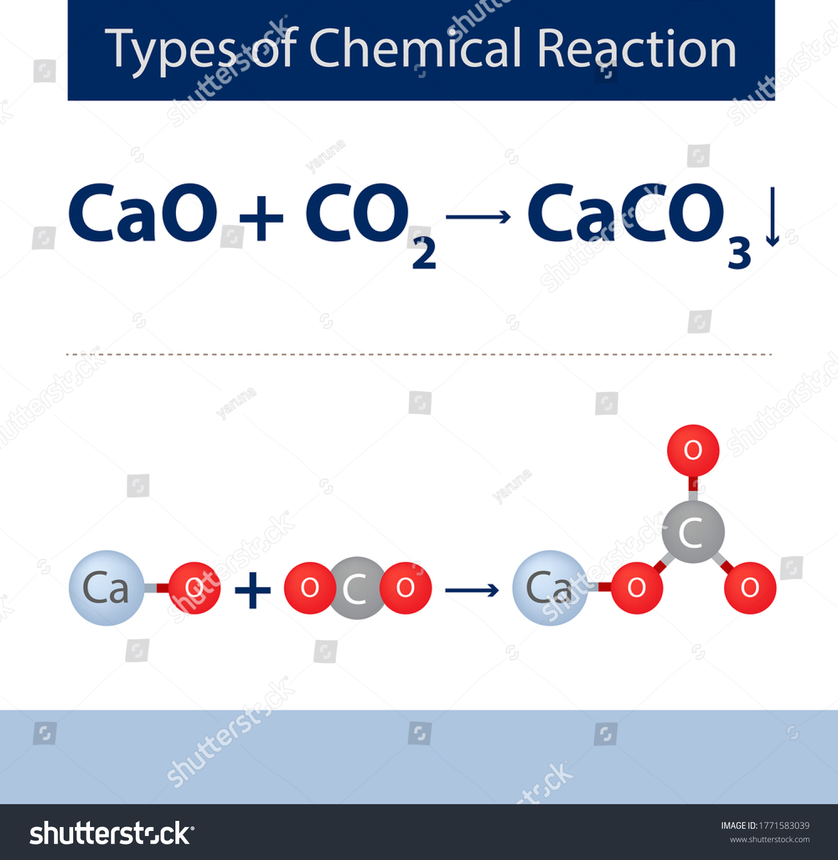
Physical Properties of Calcium Carbonate :
It is a white fluffy powder.
When it is heated at 1200 Kelvin then it decomposes to form carbon dioxide.
When it reacts with any dilute acid it liberates carbon dioxide as a byproduct.
The molecular weight of calcium carbonate is found to be 100 grams/mole
Application of Calcium Carbonate :
Calcium carbonate is employed in the paper industry as well as the pulp industry.
It is used as a pigment making higher-quality minerals.
It is also used in the construction industry.
It is used in the purification of metals such as calcination.
It is used as an additive to food products to increase their vitamin value.
It is used as a disinfectant and sewage water treatment.
Calcium carbonate acts as an antacid to treat upset stomachs and is also an acid in digestion.
Frequently Asked Questions (FAQs)
There are many uses of calcium carbonate, some of them are
Calcium carbonate is used in the purification of iron ore.
It is used in paints.
It is used in hemodialysis treatment.
It is also used as an antacid and calcium supplement.
Identification of calcium carbonate can be easily done by the addition of some dilute acids.
When acid such as sulfuric acid or hydrochloric acid is added to the compound and it liberates brisk effervescence of the carbon dioxide then it is a confirmatory test that a carbonate salt is present in the compound.
Calcium is the main source that makes our bones healthy and strong.
calcium helps our bone strength to increase and there are fewer chances of fractures. There are many fruits that have calcium present in them such as apricot and raisins.
Calcium carbonate is rich in calcium. It can be used as fertilizer to provide calcium to the plant. It also acts as the pH corrector of the soil and helps in the stabilization of the pH of the soil.
Calcium carbonate is present in many forms such as minerals, marble, Limestone, chalk, Pearl, and shells.