CaOH2 Full Form
What is the full form of CaOH2?
Calcium Hydroxide is generally called slaked lime. It is an inorganic compound named as calcium hydroxide. Ca(OH)2 is the chemical formula for it. Calcium hydroxide appears as a white powdery material in the solid state and a whitish crystal in the crystalline form. The calcium hydroxide compound is also known as hydrated, slack, pickling, and caustic lime. Water and calcium oxide are combined to create calcium hydroxide (quick lime).
Salient properties of Ca(OH)2
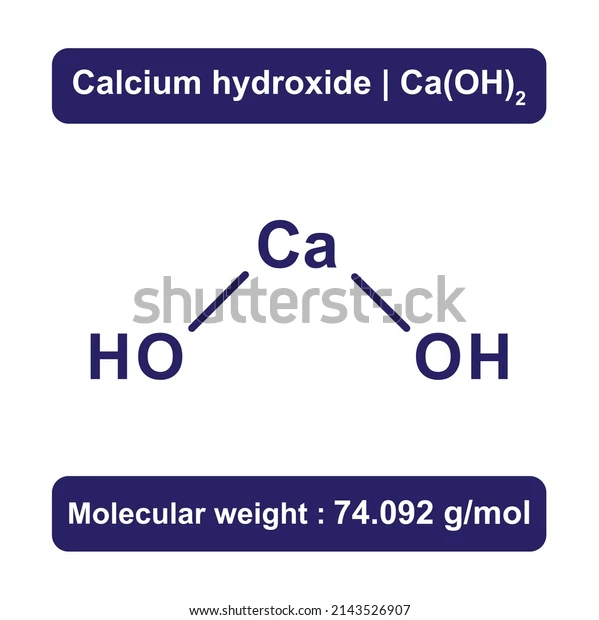
IUPAC name | Calcium hydroxide |
Chemical formula | Ca(OH)2 |
Molar mass | 74.093 g/mol |
Odour | Odourless |
Melting point | 853K |
Physical properties of Ca(OH)2
Ca(OH)2 is structured as a hexagon.
This substance isn't particularly water-soluble. However, as the temperature rises, its solubility falls. Assume that its solubility allows for a dissolving rate of 1.73 g/L at 20°C and 1.89 g/L at 0°C.
This substance typically evaporates and decomposes at temperatures near its melting point.
The solubility of calcium hydroxide is calculated to be 5.5 x 10-6.
When hydrated, it becomes a colourless white powder that is known as slaked lime.
Chemical properties of Ca(OH)2
While glycerol and acids are considerably soluble in Ca(OH)2, two water is only marginally soluble. On dissolving in water, It produces a solution that functions as a mild base until it reaches saturation (called limewater).
Salts are created when limewater and acids react.
Metals like aluminium react with and dissolve in the saturated solution of calcium hydroxide in water.
It forms calcium carbonate after reacting with carbon dioxide (CaCO3) through carbonation.
Uses of Ca(OH)2
To make lime mortar, calcium hydroxide is frequently used.
Calcium hydroxide is widely used in the sewage and water treatment industries as a flocculant. It creates a frothy charged solid that helps remove tiny particles from water for a cleaner end product.
Because it is self-regulating and does not elevate the pH too much, it is also used to treat freshwater to raise the pH so that pipelines in areas where the base water is acidic will not erode.
Slaked lime is frequently used in the food industry to prepare water for alcoholic beverages and soft drinks as well as to clear raw sugarcane or sugar beet juice due to its low toxicity and mild basic qualities.
Frequently Asked Questions (FAQs)
There are two hydrogen bond donors in Ca(OH)2.
The process of adding carbon dioxide gas to a beverage in order to give it a sparkle and a tangy flavour and to keep it from spoiling is called Carbonation.
The main ingredients are sand- or lime-based aggregate, water, and lime mortar. Today, the preservation of existing historic structures or the traditional reconstruction of new ones primarily involves the use of lime mortar.
Calcium hydroxide in food-grade form is generally secure. It can cause calcium hydroxide poisoning if you consume it while working with industrial-grade calcium hydroxide. A severe injury or death could result from this.