CH2 Full Form
What is the full form of CH2?
Methylene is a radical which has 2 electrons in the free state and it is very reactive. Methylene has the chemical formula CH2 which is also known as carbene. Methylene resembles chloroform in terms of appearance and smell but differs from it in terms of boiling point and specific gravity. Early in the 19th century, methylene, which is wood alcohol, CH3OH was used to refer to methanol. Methylene refers to wine made from wood because it is made up of the words 'methe' and 'hyde', the latter of which is the Greek word for wood.
1. Properties of Methylene
CH2 | Methylene |
Density | 0.98 g/mL at 25 °C |
Molar Mass/ Molecular Weight | 14.0266 g/mol |
Boiling Point | 39.8° C at 760 mm Hg |
Chemical Formula | CH2 |
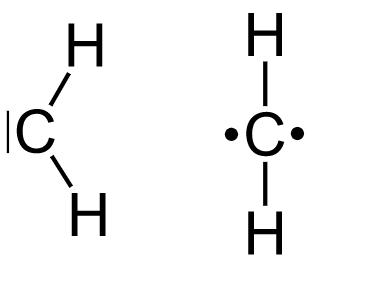
2. Structure of Methylene
In organic chemistry, methylene refers to any component of a molecule that consists of two hydrogen atoms bound to a carbon atom. Two bonds are used to join this portion of the molecule to the remaining portion of the molecule to satisfy the carbon atom's valency. The two bonds in the group can be represented by the symbol CH2. A different illustration might be -CH2-
3. Discovery and preparation
Gerhard Herzberg and Jack Shoosmith were the ones to first synthesise and spectroscopically characterise the compound diazomethane. The discovery was made using Flash photolysis.
Ketene (ethenone) (CH2=CO), diazomethane (linear CH2=N2), diazirine (cyclic [-CH2-N=N-]), and diiodomethane are examples of compounds with a methylidene group that can be decomposed under favourable conditions to produce methylene (I-CH2-I). Photolysis, photosensitized chemicals (like benzophenone), and thermal decomposition can all have an impact on the decomposition.
4. Physical properties of Methylene
Odour | No Odour |
Appearance | Colourless |
Covalent Bonded Unit | 1 |
Molar Entropy |
|
Heavy Atom Count | 1 |
Solubility | Soluble in water |
5. Chemical properties of Methylene
Methane, a saturated hydrocarbon, is created when methylene and hydrogen in the atmosphere react. Below is the chemical equation.
![]()
Methylene is easily oxidised, producing water and carbon monoxide in the process. Below is the chemical equation.
![]()
6. Uses of Methylene
In many paint removers, including industrial and household paint strippers, methylene as methylene chloride is the active component.
In both human and veterinary medicine, methylene is used as methylene blue for a variety of therapeutic and diagnostic procedures.
Chemical DNA has been detected using cyclic voltammetry using methylene as an electroactive probe.
Additionally used to treat some types of genetic methemoglobinemia and drug-induced methemoglobinemia.
Frequently Asked Questions (FAQs)
The main distinction between the methyl and methylene groups is the presence of one carbon atom in the methyl group, which is bonded to three hydrogen atoms, as opposed to one carbon atom in the methylene group, which is bonded to two hydrogen atoms.
No, Alkenes, like alkanes, form a homologous series of molecules that gain molecular weight by incorporating methylene units (–CH2–). A molecule is referred to as a terminal alkene if its double bond is at the end of it.
Any area of a molecule made up of two hydrogen atoms linked to a carbon atom, which is connected to the rest of the molecule by two single bonds, is referred to as a methylene group in organic chemistry.
The group with two -I or -R groups on either side is the group that contains active methylene. The hydrogen in this group, CH2, is therefore very acidic. Let's consider acetylacetone as an illustration.
A carbon, which also has three additional hydrogen bonds bound to hydrogen, is where the methyl protons are attached. Methylene's protons are connected to a carbon, which is connected to another carbon, fluorine, and yet another carbon. NMR Spectrum No. 2 Different proton groups result in two signals.