Chemistry - Definition, Types, Facts, Chapters, FAQs
Chemistry is the scientific study of matter—anything that has mass and occupies space—and it explores how different substances are structured, what they are made of, and how they behave. This field examines physical features like color, density, melting and boiling points, as well as chemical behavior such as reactivity and acidity. Chemistry also looks at the transformations that occur during physical and chemical reactions, including how new substances form or change.
This Story also Contains
- What is Chemistry
- History of Chemistry
- Scope of Chemistry
- Branches of Chemistry
- Major Branches of Chemistry
- 1. Organic Chemistry
- 2. Inorganic Chemistry
- 3. Physical Chemistry
- Real-Life Applications And Relevance Of Chemistry
- NCERT Resources For Students
Often called the central science, chemistry connects physics and biology by explaining what happens at the molecular and atomic level. It provides the foundation for understanding biological processes like metabolism and molecular biology, as well as physical phenomena involving energy, bonding, and atomic interactions. Since chemistry bridges these scientific areas, it plays a key role in fields such as medicine, environmental science, materials science, and pharmacology. Overall, chemistry helps us understand and predict the molecular basis of natural and technological phenomena.
What is Chemistry
Chemistry is a key branch of science that focuses on the study of matter, its properties, and how different substances interact and change. It helps us understand what materials are made of and how chemical reactions work in everyday life. Through chemistry, scientists develop useful products such as medicines, fertilizers, and clean energy solutions. The field of chemistry is divided into major branches including organic, inorganic, physical, analytical, and biochemistry. Each branch explores a different aspect of chemical science, helping solve real-world problems in industries like healthcare, agriculture, and environmental science.
History of Chemistry
The history of chemistry is full of important discoveries that shaped how we understand matter and its changes. In ancient times, people practiced alchemy, trying to turn common metals into gold. Although they didn’t succeed, these early experiments laid the foundation for modern science. Over time, chemistry evolved into a structured science with clear principles. One of the key figures in this development was Antoine Lavoisier, known as the "Father of Modern Chemistry." He named the element oxygen and discovered its vital role in the process of combustion, which greatly advanced our understanding of chemical reactions.
Scope of Chemistry
Chemistry is a wide-ranging science with applications in many important fields. It plays a key role in industries such as pharmaceuticals, agriculture, environmental science, material science, energy, and food technology. Chemistry helps in creating new medicines, improving crop production, developing eco-friendly materials, and finding clean energy solutions. In academics, chemistry offers strong career opportunities in research and development, where scientists discover and innovate new technologies. It also has a major role in education, with teaching positions available in schools, colleges, and universities. With its broad scope, chemistry continues to impact both science and everyday life.
Branches of Chemistry
Here we discuss briefly the branches of chemistry. There are five main branches of chemistry and those are:
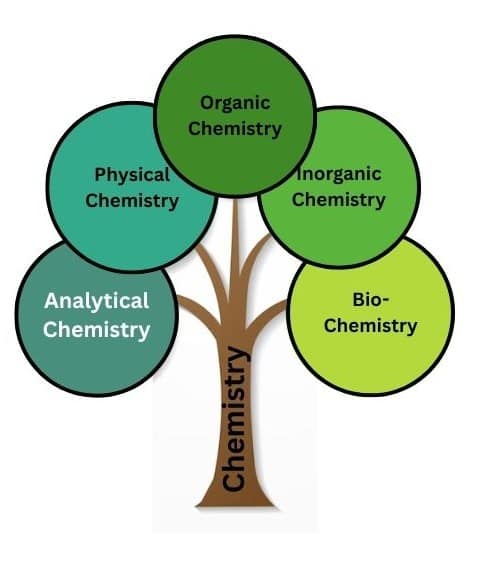
1) Physical Chemistry
Physical chemistry is the branch of chemistry in which we study the principles of physics combined with chemistry. In physical chemistry, we study the physical properties of molecules and the changes they undergo during chemical reactions. In this, we focus on the behavior of matter at the molecular levels, applying numerical concepts in kinetics, mechanics, and thermodynamics.
2) Organic Chemistry
Organic chemistry is the branch of chemistry in which we study the carbon-containing compounds. In organic chemistry, we focus on the structure of carbon-containing compounds, reactions related to them, and their synthesis. In organic chemistry, there are a wide variety of compounds including hydrocarbons(made up of carbon and hydrogens only), and other compounds containing nitrogen, oxygen, sulphur, and halogens.
3) Inorganic Chemistry
Inorganic chemistry is a branch of science that deals with the study of inorganic compounds. Inorganic chemistry includes a wide variety of compounds that are not part of organic chemistry. We study the metals, non-metals, coordination compounds, organometallics, and materials in this field.
4) Analytical Chemistry
Analytical chemistry is a branch of chemistry that focuses on identifying and measuring the components of substances. It involves both qualitative analysis, which tells us what a substance contains, and quantitative analysis, which tells us how much of each component is present. This field uses various scientific techniques to separate, detect, and quantify chemical compounds in different types of samples. Analytical chemistry is essential in industries like pharmaceuticals, food safety, environmental testing, and forensic science. By understanding the exact composition of materials, analytical chemistry helps ensure product quality, safety, and compliance with regulations.
5) Biochemistry
Biochemistry is the branch of chemistry that is related to the chemical reactions in the living system. In this, the application of both chemistry and biology are combined to understand the molecular reaction with the biological functions.
Major Branches of Chemistry
There are mainly three branches of chemistry. Here we discuss the chapters and topics in organic, inorganic and physical chemistry.
1. Organic Chemistry
Chapters 1: Some Basic Principles And Techniques
Some Basic Principles And Techniques is the chapter of organic chemistry is generally called general organic chemistry in this we study all the effects and intermediate, reaction mechanisms etc.
Here are some topics in this chapter:
- Organic Compounds -Classification of Organic Compounds
- Functional Group
- IUPAC Nomenclature Of Organic Chemistry
Chapter 2: Hydrocarbons
Hydrocarbons are organic compounds that are composed of carbon and hydrogen molecules. And occur naturally on the earth. Hydrocarbons are the basis of various substances such as natural gas, petroleum, coal, and crude oil.
Some topic of this chapter are:
Chapter 3: Haloalkanes And Haloarenes
Haloalkanes And Haloarenes are a group of hydrocarbons in which the halogen atom replaces one hydrogen atom. In this chapter on organic chemistry, we study the preparation, physical properties, chemical properties, uses, and other halogen-containing compounds.
Some topic of this chapter are:
Chapter 4: Alcohols, Phenols, And Ether
Alcohols, Phenols, And Ether is a chapter of organic chemistry in which we study the preparation, properties, and reaction of Alcohol. Alcohol is a hydrocarbon in which one hydrogen is replaced by an OH group.
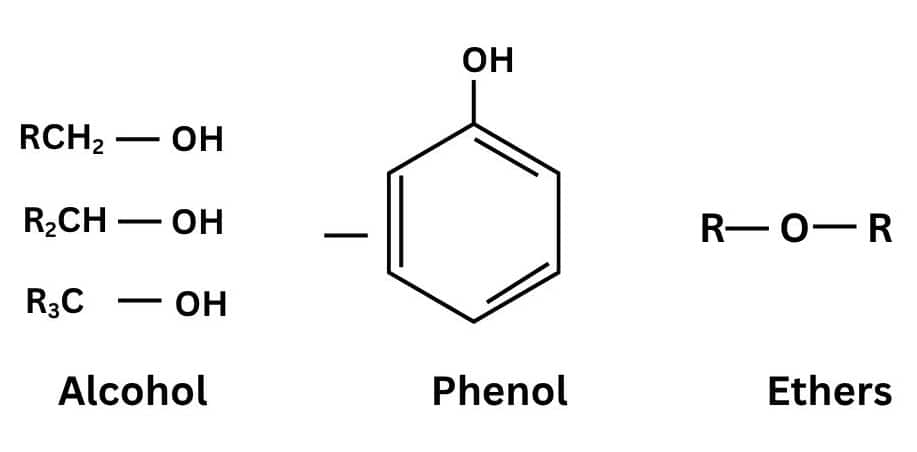
Some topic of this chapter are:
Chapter 5: Aldehydes, Ketones And Carboxylic Acid
Aldehydes, Ketones And Carboxylic Acid are organic compounds that contain a functional group of CHO, CO, and COOH. Aldehyde and Ketone have a lower boiling point than alcohol and carboxylic acid has a higher boiling point than alcohols.
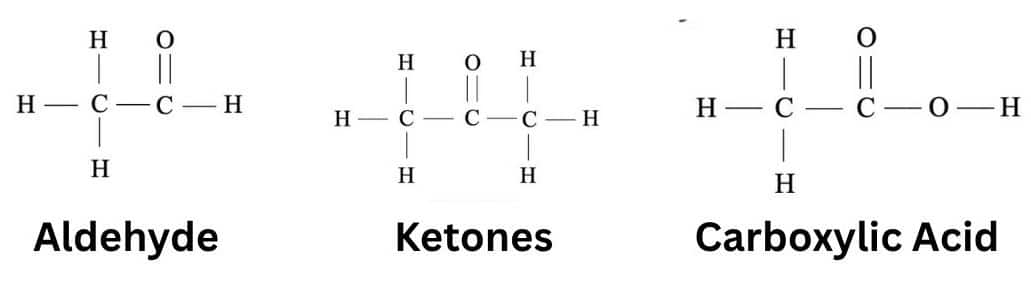
Here are some topics of this chapter:
Chapter 6: Amines
Amines are nitrogen-containing organic compounds that are generally derived from ammonia by replacement of one more hydrogen atom by an alkyl group.
.jpg)
Here are some topics of Amines:
Chapter 7: Biomolecules
In Biomolecules, we study the carbohydrates,types of carbohydrates like glucose and fructose, Vitamins, Proteins and Amino acids.
 Some topics of Biomolecules are:
Some topics of Biomolecules are:
Chapter 8: Polymers
Polymers are natural and synthetic substances that are composed of various, monomer units. In this chapter, we study the preparation, properties, and uses of various polymers.
Some topic of this chapter are:
Chapter 9: Environmental Chemistry
Environmental Chemistry is also a part of organic chemistry in this we generally study the chemical interaction with the environment like with air, water, soil. How this the environment affect with the chemical or chemical activities.
Here are some topics of this chapter:
Chapter 10: Chemistry In Everyday Life
In Chemistry In Everyday Life we study how chemistry is influenced by our life. how we use chemistry in our daily life process we use chemicals at almost every stage of our life without even realizing.
Here are some topic of this chapter:
2. Inorganic Chemistry
Chapter 1: Classification Of The Elements And Periodicity Of Properties:
Classification Of Elements And Periodicity Of Properties is the chapter of inorganic chemistry in which we study the elements and their arrangement in the periodic table and the trends of the elements.
Some topics of this chapter are:
Chapter 2: Chemical Bonding And Molecular Structure:
In Chemical Bonding And Molecular Structure we study the three-dimensional structure of the molecules and their molecular geometry and shapes of the orbital.
Some topics of this chapter are:
- Chemical Bonding
- Ionic Bond Or Electrovalent Bond
- Bond Parameters - Bond Order, Angle, Length, And Energy
Chapter 3: S- Block Elements
S- Block Elements are those elements that are present in the first two groups of the periodic table. In this chapter, we study the properties of those elements whose last electron enters the s-subshell.
Here are some topics of this chapter:
Chapter 3:Coordination Compounds
Coordination Compounds are those compounds that consist of arrangements of anions and neutral molecules bonded to the central metal atoms or ions, which are generally metallic and bound to the ligands to orm the coordination compound.
Here are some topics of this chapter:
Chapter 4: Redox Reaction
When oxidation and reduction occur simultaneously in the reaction that reaction is called Redox Reaction. This is a chapter on inorganic chemistry in which we study oxidation state and oxidation number.
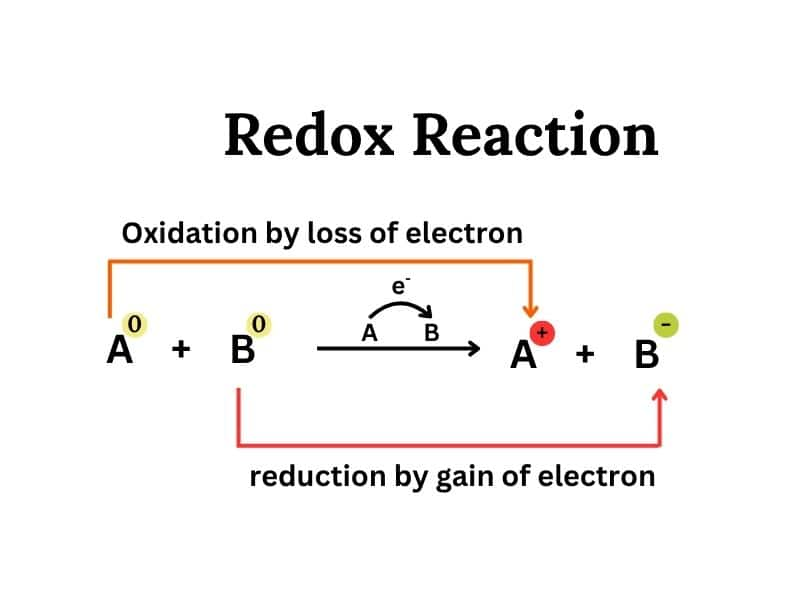 Here are some topics of this chapter:
Here are some topics of this chapter:
Chapter 6: Hydrogen
Hydrogen is the simplest member of the chemical element family. It is the lightest element and at the standard condition it is diatomic gas.
Here are some topics of this chapter:
Chapter 7: General Principle And Processes Of Isolation
General Principles And Processes Of Isolation is generally Metallurgy it is the chapter of inorganic chemistry in which we study how to extract substances like diamonds, or metals from their ores. Metal is found in the form of ores.
Here are some topics of this chapter:
Chapter 8: D- And F- Block Elements
The elements whose electron enters into the d- subshell and the elements whose last electron enters into the penultimate shell are called D- And F- Block Elements. D-block elements are called transition elements and f-block elements are lanthanoids, and actinoids.
Here are some topics of this chapter:
Chapter 9: P- Block Elements
P- Block Elements are generally called representative elements. It includes metals, non-metals, and metalloids. These are situated on the right side of the periodic table.
.png)
Here are some topics of this chapter:
3. Physical Chemistry
Chapter 1: Some Basic Concepts Of Chemistry
Some Basic Concepts of Chemistry is the chapter of physical chemistry in which we study basic terms of chemistry which are used to measure the quantities of the compounds in a minor amount.
Here are some topics of this chapter:
Chapter 2: Structure Of Atom
Structure Of Atom is the chapter of physical chemistry in this we generally study the arrangement of electron in the atom.
Some topic of this chapter are:
Chapter 3: States Of Matter
States Of Matter exists in various forms like gaseous state, solid state, and liquid state. It is generally the distinct form in which, matter can exist.
Here are some topics of the this chapter are:
Chapter 4: Thermodynamics
Thermodynamics is the study of heat, work done, and the relation between heat and temperature.
Here are some topics of this chapter:
Chapter 5: Equilibrium
In Equilibrium, we cover chemical equilibrium and ionic equilibrium both. The equilibrium is a steady state when the reaction occurs at the same speed in both forward and backward directions.
Some topics of this chapter are:
Chapter 6: Solutions
A Solution is a heterogeneous mixture formed by the solute dissolved in the solvent. In this chapter, we study the properties of the solution and many more.

Here are some topics of this chapter:
Chapter 7: Electrochemistry
Electrochemistry is the part of physical chemistry in this chapter we study the cell, electrolytic cell, galvanic cell, about the battery and electrolyte and conductivity.
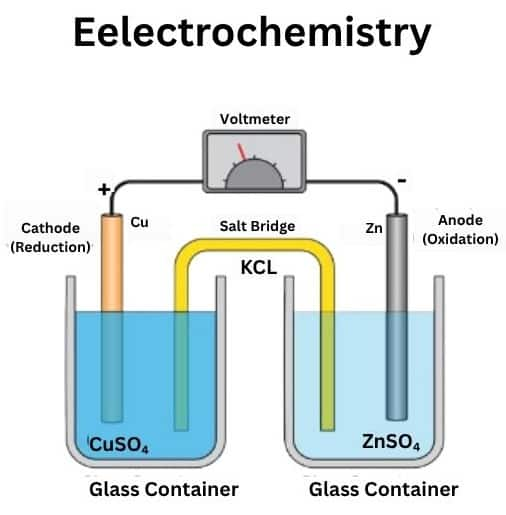
Some topics of this chapter are:
Chapter 8: Chemical Kinetics
Chemical Kinetics is the study of the rate of reaction. The rate at which the reaction takes place when the reactant is turned into the product. In this, we focus on the dynamics and time of the reaction, not the temperature.
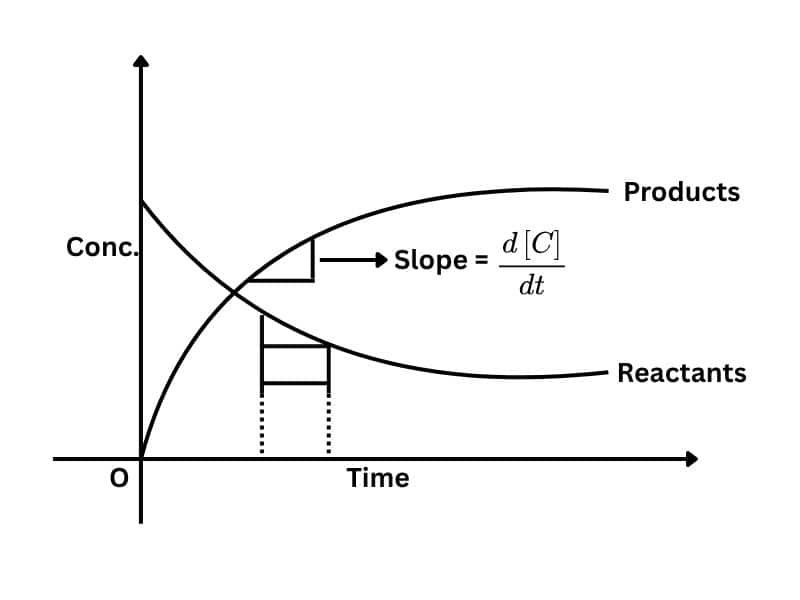
Here are some topics of this chapter:
Chapter 9: Surface Chemistry
Surface Chemistry is the chemical and physical phenomenon that occurs in between two forms including solid-liquid interlinking, solid-gas interlinking, and liquid-gas interlinking.
Some topics of this chapter are:
Real-Life Applications And Relevance Of Chemistry
Chemistry is related to many aspects of our real life such as:
-
Food & Cooking: Cooking involves chemical transformations—like browning foods (Maillard reaction), eggs turning solid when heated, and sugars caramelizing to add flavor.
-
Fermentation: Microbes convert sugars into alcohol, acids, or gases, creating products like bread (CO₂ for rising), beer, wine, yogurt, and pickles.
-
Medicine: Chemistry underlies the creation of medicines, antibiotics, and vaccines, guiding how drugs are designed, formulated, and safely delivered.
-
Industrial Chemistry: From textiles and plastics to detergents and colorants, chemistry powers manufacturing processes and product improvements.
-
Everyday Products: Soaps, cleaners, and preservatives rely on chemical principles—surfactants remove grease, acids prevent spoilage, and catalysts speed up production.
NCERT Resources For Students
Careers360 offers a wide range of resources for Students like Ncert books, Syllabus, sample papers, NCERT solutions, Exampler Solutions and many more things.
| Chemistry Syllabus | |
| Chemistry NCERT Books | |
| NCERT Exemplar | |
| Chemistry Sample Paper | |
| CBSE Chemistry Notes | |
| Chemistry NCERT Solutions | |
| Chemistry Formulas | Chemistry Formulas PDF |
Frequently Asked Questions (FAQs)
Solutions, Electrochemistry, Chemical Kinetics, d -and f -Block Elements, Coordination Compounds, Haloalkanes and Haloarenes, Alcohols, Phenols and Ethers, Aldehydes, Ketones and Carboxylic Acids, Organic Compounds Containing Nitrogen, Biomolecules.
In chemistry, there are three parts Organic, inorganic, and physical chemistry.
There are 16 chapters given in the NCERT book class 12.
There are changes around us such as sugar dissolving in water, the lake freezing in winter etc. One change is what chemistry scientists call chemical reactions, and others are not. Chemical changes occur when new products are made that are different from what we started with.
Cooking eggs, for example, are an example of a chemical change; egg white and egg yolk change from liquid to solid. Heat makes the egg protein stronger.
Aqua Regia is Kings Water; this is because it has enough power to dissolve gold - the king of metals. It is prepared by mixing three parts hydrochloric acid and one part nitric acid, but in the old days it was prepared for mixing and roasting salt. For example, we can mix two parts niter and one part Sal. Ammonia and distilled at high temperatures from Aqua Regia.
"Sulfuric acid" is also called the king of acids and "Nitric acid" is known as the Queen of acids.
Chemistry is the science that studies the atoms and molecules and their structures. Everything is basically made up of atoms and also molecules. There are basically five major branches of chemistry, namely.
Natural chemistry
Inorganic chemistry
Physical chemistry
Biochemistry
Analytical chemistry
Organic chemistry is simply the study of carbon dioxide. Organic chemistry is important because it is the study of life and all life-related chemical reactions. Organic chemistry initially involves the study of chemicals that can be found in living things.
About 7 million organic compounds are currently known while only 1.5 million compounds are known. This large number of organic compounds emanate from the unique carbon field.
Blue vitriol is also known as blue copperas. The word vitriol blue has a strong and clear meaning. Copper sulfate and chemical formula CuSO4.5H2O. The chemical name for vitriol blue is Copper (II) Sulfate Pentahydrate. This salt occurs in the form of a deep rhomboidal prism of deep blue color, with an overly complex taste and done in style.
Similarly, "Green Vitriol" refers to Ferrous Sulphate.