Alloy - Definition, Examples, Types of Alloys with FAQs,
Alloys are substances created from the mixing of two or more metals. The properties of alloys are frequently dissimilar to the qualities of their constituent components. When compared to pure metals, alloys frequently have greater strength and hardness. Red gold, which is made by combining copper and gold, is an example of an alloy. White gold, which is made by combining silver and gold, is another major gold alloy.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
In this article, we cover the concept of alloys. which is a very important topic in the chapter The Solid States from the Board exam point of view and also for the JEE Mains Exam, NEET Exam, and many other entrance exams like SRMJEE, BITSAT, WBJEE, BCECE, and more
Alloys are known to feature metallic bonding. For almost all practical applications, the constituents of an alloy are measured in terms of their mass percentage. However, for some scientific studies, the components of alloys are measured in terms of their atomic fractions. It is important to note that alloys can be classified into two categories based on the arrangements of atoms in their respective lattices: substitutional alloys and interstitial alloys. It can also be noted that alloys can be classified based on the total number of phases present. For example, homogeneous alloys feature only one phase and heterogeneous alloys feature two or more phases.
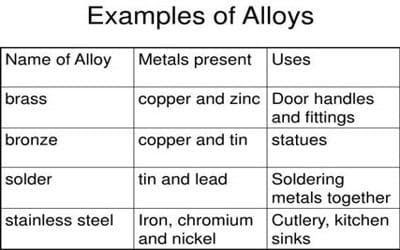
Examples of alloys
Babbitt metal, commonly known as bearing metal, is an alloy used primarily as a bearing surface in simple bearings. Isaac Babbitt, an American inventor, created this alloy in the year 1839. The following is a typical Babbitt metal composition:
90 percent tin (Sn)
7 percent antimony (Sb)
Copper (Cu) accounts for 3% of the total.
One of the most appealing properties of this alloy is it has minimal friction with steel.
The chemistry of alloys
A strong electron microscope can reveal the atoms inside a metal. Then the atoms are grouped in a regular arrangement called a crystalline lattice.
Related Topics,
- Preparation Properties and Uses of Sodium Chloride
- Bravais Lattice
- Crystal Lattices and Unit Cells
- Schottky Defect
Types of alloys
substitution Alloy
A replacement alloy is formed when the atoms of the alloying agent replace the atoms of the primary metal. Only if the atoms of the base metal and the alloying agent are nearly the same size can an alloy like this form. In most replacement alloys, the constituent elements are close in the periodic table to one another. Brass, for example, is a copper-based substitution alloy in which zinc atoms replace 10–35 percent of the copper atoms that are ordinarily present. Because copper and zinc are near in the periodic table and contain atoms that are roughly the same size, brass works well as an alloy.
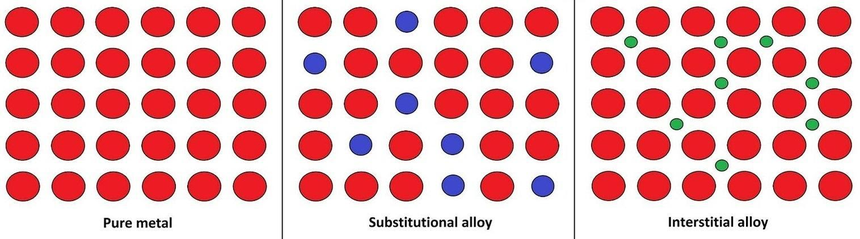
Interstitial Alloy
Alloys with interstitial spaces
Alloys can also occur when the alloying agent or agents have atoms that are significantly smaller than the parent metals. The agent atoms then slide between the gaps of metal forming an interstitial alloy. One of the examples of an interstitial alloy is steel where a small number of carbon atoms slide between the massive atoms in an iron crystalline lattice.
Bell Metal
Bell metal is an alloy that is predominantly used in the manufacture of bells and other similar instruments (thus the name). It is a type of bronze that contains a significant quantity of tin in its composition. Bell metal is an alloy of copper and tin. Bell metal is often made up of the following elements:
Copper (Cu) accounts for 78 percent of the total.
Tin (Sn) accounts for 22% of the total.
Cooking and dining utensils are also made of this alloy. It can also be found in some styles of home decor.
Bronze
Bronze is a copper and tin alloy. Medals, coins, trophies, hefty gears and tools, and several types of electrical devices all employ it. Bronze is an alloy of elements copper and tin.
Bronze composition:
Copper (Cu) accounts for 75% of the total.
Tin (Sn) - contains up to 12%
Manganese, aluminium, zinc, nickel, silicon, phosphorus, and arsenic are examples of other elements.
It should be noted that the strength of the bronze varies depending on the components employed in the alloying process. It should be noted that bronze has a far higher hardness than pure copper. It's also more ductile and machinable than pure copper.
Nichrome
Nichrome is an alloy made mostly of nickel and chromium, as the name implies. It's not uncommon for nichrome alloys to contain iron and other elements as well. The following is a typical nichrome alloy composition:
Nickel (Ni) contains 80-85% chromium (Cr) Iron and other elements account for 15-20% of the total.
Resistance wires are the most common use of nichrome. Certain electrical equipment, such as space heaters and bread toasters, employ it as the heating element. It's also worth noting that nichrome alloys are used in certain types of dental fillings.
Steel
It is largely an iron and carbon alloy. Different varieties of steel, on the other hand, are known to have various levels of carbon, as well as other elements (such as chromium, manganese, sulphur, phosphorus, nickel, copper, and molybdenum). Iron makes up the majority of steel's composition (it typically accounts for at least 75% of the alloy's weight). Depending on the type of steel, it may also contain variable levels of carbon and other metals. Stainless steel, for example, is made up of the following elements:
Iron (Fe) content is between 85 and 88 percent
A chromium (Cr) content of at least 10.5 percent is required
Carbon (C) emissions are less than 1.2 percent
Also read -
Frequently Asked Questions (FAQs)
Alloys are metal alloys or metal alloys with additional elements. Certain other metals/elements can be added to metals in certain ratios to impart certain properties or to strengthen some of their existing properties, resulting in alloys. Pure aluminium, for example, is a rather soft metal. Copper is also a soft metal. When aluminium is alloyed with copper, however, the resulting alloy has a far higher strength than the parent metals.
The following are some commercially important alloys:
steel, Nichrome, Bronze, Brass, Duralumin, solder
The following is a list of five common alloying elements.
Chromium
Vanadium
Molybdenum
Nickel
Manganese
A blend of two or more elements, at least one of which is a metal, is known as an alloy. Some alloys, such as brass and bronze, are presumably familiar to you. Brass is a copper and zinc alloy. Bronze is a copper and tin alloy.
Steel is formed of iron and carbon alloy.
Common types of alloys include:
- Steel: An alloy of iron and carbon, often with other elements to improve strength and corrosion resistance.
- Brass: An alloy of copper and zinc, known for its malleability and acoustic properties.
- Bronze: An alloy primarily of copper and tin, known for its resistance to corrosion and wear.
- Aluminum Alloys: Mixtures of aluminum with elements like copper, magnesium, or silicon, are used in various applications due to their lightweight and strength.
Alloys are used in a wide range of applications, including:
- Construction: Steel is commonly used in building materials and structures.
- Aerospace: Aluminum alloys are used for aircraft due to their lightweight and strength.
- Automotive: Various alloys are used for engine components, wheels, and body panels.
- Marine: Bronze and other corrosion-resistant alloys are used in shipbuilding and marine applications.
The main advantages of using alloys include:
- Improved Strength: Alloys often possess enhanced tensile strength and hardness compared to their component metals.
- Corrosion Resistance: Many alloys are designed to resist rust and chemical degradation.
- Ductility and Malleability: Alloys often maintain or improve the workability of metals, allowing for easier shaping and manufacturing.
- Cost-Effectiveness: Using cheaper metals or combining metals can help reduce costs while enhancing performance.
The common examples of alloys are brass, stainless steel, bronze, aluminum alloys, copper-nickel alloys, titanium etc.
Also Read
16 Dec'24 11:31 PM
13 Nov'24 05:34 PM
13 Jul'22 10:37 AM
13 Jul'22 10:33 AM
30 Jun'22 03:58 PM