Ammonia - Structure, Preparation, Uses, FAQs
Ammonia is a colorless gas with a pungent odor, having chemical formula $\mathrm{NH}_3$ is commonly used as a fertilizer and in various industrial applications. Hydrogen and nitrogen are its components. It is also known as ammonium hydroxide in its aqueous form. It can be dangerous in concentrated form. In this article, we will learn about the characteristics, structure, preparation methods, physical and chemical properties, and important uses of ammonia. Understanding these concepts is essential for students preparing for JEE Main and NEET. Some solved questions of ammonia are also given in this article.
This Story also Contains
- Characteristics of Ammonia Gas - $\mathrm{NH}_3$
- Structure of Ammonia $\mathrm{NH}_3$
- Preparation of Ammonia
- Uses of Ammonia ($\mathrm{NH}_3$)
- Some Solved Examples
Characteristics of Ammonia Gas - $\mathrm{NH}_3$
| $\mathrm{NH}_3$ |
Ammonia |
|
Molecular Weight/ Molar Mass |
17.031 g/mol |
|
Density |
0.73 kg/m³ |
|
Ammonia boiling point |
-33.34 °C |
|
Melting Point |
−77.73 °C |
The compound ammonia gas is known as a weak base since it forms ammonia salts when combined with a variety of acids. The result of the reaction between ammonia gas and hydrochloric acid is ammonium chloride. The ammonium cation NH4+ is found in all ammonia salts produced through such acid-base reactions. Liquid ammonia has also been found to possess weak acidic properties and, therefore, can be considered an amphoteric compound. With some alkali metals and alkaline earth metals, ammonia forms amides due to its acidic qualities. The NH3 molecule is also known to undergo self-dissociation once it is dissolved in water. Ammonia molecules undergo molecular autoionization, which forms their conjugate bases (NH2–) and acids (NH4+). Below is an illustration of the structure of the ammonium cation.
Structure of Ammonia $\mathrm{NH}_3$
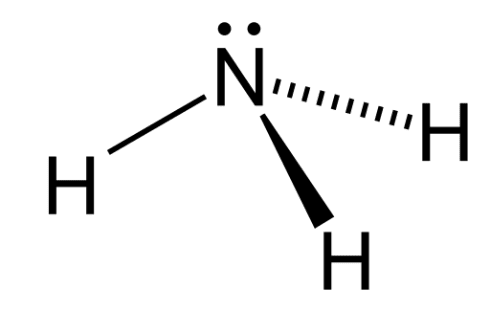
Ammonia (NH₃) has a trigonal pyramidal structure, formed by one nitrogen atom and three hydrogen atoms. The nitrogen atom is sp³ hybridized, with three of its hybrid orbitals forming σ (sigma) bonds with hydrogen atoms, and the fourth containing a lone pair of electrons.
Because of this lone pair, the molecule is not planar the lone pair repels the N–H bonds, giving ammonia its pyramidal shape.
Preparation of Ammonia
Sodium hydroxide or calcium hydroxide are powerful alkalis which are used to prepare ammonia in the laboratory.
$2 \mathrm{NH}_4 \mathrm{Cl}+\mathrm{Ca}(\mathrm{OH})_2 \rightarrow \mathrm{CaCl}_2+2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{NH}_3(\mathrm{~g})$
Alternatively, the gas can be made by heating concentrated ammonium hydroxide.
Under high pressure and the presence of a catalyst, nitrogen and hydrogen are directly combined to produce ammonia in the Haber Process.
Uses of Ammonia ($\mathrm{NH}_3$)
-
Fertilizing crops with it produces higher yields
-
As a cleaner, stainless steel and glass can be cleaned with NH3 inside a home
-
Food products containing it provide antimicrobial properties
-
There are many industries that use it for fermentation
-
The refrigerant used in refrigeration is this
-
The pH of fermentation processes must be adjusted
-
Hence, it neutralizes emissions of diesel engine pollutants such as nitrogen oxides
-
For rocket engines, it is used as a fuel
-
In textile industries, it is used
-
For manufacturing synthetic fibres like rayon and nylon
Also Read :
Some Solved Examples
Question 1: Identify the products when chlorine react with excess ammonia
1) $\mathrm{NCl}_3$ and HCl
2) $\mathrm{NCl}_3$ and $\mathrm{N}_2$
3) (correct) $\mathrm{NH}_4 \mathrm{Cl}$ and $\mathrm{N}_2$
4) $\mathrm{NCl}_3$ and $\mathrm{NH}_4 \mathrm{Cl}$
Solution:
As we learned
Reaction with Ammonia in excess Chlorine -
$\mathrm{NH}_3+3 \mathrm{Cl}_2 \rightarrow \mathrm{NCl}_3+3 \mathrm{HCl}$
wherein
$\mathrm{NCl}_3$ is an explosive
$8 \mathrm{NH}_3+\mathrm{cl}_2 \rightarrow 6 \mathrm{NH}_4 \mathrm{cl}+\mathrm{N}_2$
Hence the answer is the option (3).
Question 2: Which property of $\mathrm{NH}_3$ is shown in the following reaction-
$\mathrm{NH}_3+\mathrm{CuO} \rightarrow$
1) (correct) Reducing property
2) Oxidising property
3) Basic Property
4) Acidic property
Solution:
As we learn
Ammonia as a reducing agent -
Reduces heated CuO and $\mathrm{Cl}_2$
wherein
$\begin{aligned}
& 2 \mathrm{NH}_3+3 \mathrm{CuO} \xrightarrow{\Delta} 3 \mathrm{Cu}+3 \mathrm{H}_2 \mathrm{O}+\mathrm{N}_2 \\
& 2 \mathrm{NH}_3+3 \mathrm{CuO} \rightarrow 3 \mathrm{Cu}+\mathrm{N}_2+3 \mathrm{H}_2 \mathrm{O}
\end{aligned}$
$\mathrm{NH}_3$ is a reducing agent while CuO is an oxidising agent.
Hence, the answer is the option (1).
Question 3: Which is a very good solvent for alkali metals.
1) Liquid $\mathrm{SO}_2$
2) Liquid Ozone
3) (correct) Liquid $\mathrm{NH}_3$
4) Liquid $\mathrm{H}_2 \mathrm{O}$
Solution:
As we learn
Manufacture of ammonia by Calcium Cyanamide -
$\mathrm{CaCN}_2$ is hydrolysed with superheated steam
wherein
$\begin{aligned}
& \mathrm{CaC}_2+\mathrm{N}_2 \xrightarrow{\Delta} \mathrm{CaCN}_2+\mathrm{C} \\
& \mathrm{CaCN}_2+3 \mathrm{H}_2 \mathrm{O} \xrightarrow{450 \mathrm{~K}} \mathrm{CaCO}_3+2 \mathrm{NH}_3
\end{aligned}$
liq. $\mathrm{NH}_3$ is a very good solvent for alkali metals and alkaline earth metals.
$\text { example : } \mathrm{Na}+(x+y) \mathrm{NH}_3 \rightarrow\left[\mathrm{Na}\left(\mathrm{NH}_3\right)_x\right]+\left[e\left(\mathrm{NH}_3\right)_y\right]$
Hence, the answer is option (3).
Question 4: Which gas is produced by the decay of nitrogenous organic matter, like urea:
1) NO
2) NO2
3) N2O
4) (correct) NH3
Solution:
As we learn
Production of ammonia -
$\begin{aligned}
& \mathrm{CaCN}_2+3 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CaCO}_3+2 \mathrm{NH}_3 \\
& \mathrm{AlN}+3 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Al}(\mathrm{OH})_3+\mathrm{NH}_3
\end{aligned}$
Decay of urea produces ammonia,
$\mathrm{NH}_2 \mathrm{CONH}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow\left(\mathrm{NH}_4\right)_2 \mathrm{CO}_3 \rightleftharpoons 2 \mathrm{NH}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$
Hence, the answer is option (4).
Question 5: The hybridization and shape of $\mathrm{NH}_3$ molecule are respectively.
1) $s p^2$, tetrahedral
2) $s p^3$, tetrahedral
3) $s p^2$, Planar
4) (correct) $s p^3$, pyramidal
Solution:
As we learn
Structure of Ammonia -
N atom is sp3 hybridized with a love pair of electrons. Pyramidal in shape.
 N:
N:
$s p^3$ hybrid
Shape: Pyramidal
Hence, the answer is option (4).
Practice more questions with the link given below:
Frequently Asked Questions (FAQs)
Ammonia formula is NH₃. The ion NH₄⁺ is called the ammonium ion.
Yes, NH₃ (ammonia) is a colorless gas with a distinct, pungent odor at room temperature. It is commonly used in fertilizers and as a cleaning agent.
The structure of NH₃ (ammonia) is trigonal pyramidal, with the nitrogen atom at the top and three hydrogen atoms at the base. The nitrogen atom has a lone pair of electrons, which contributes to the molecule's shape and polarity. The bond angle between hydrogen, nitrogen, and hydrogen atoms (H-N-H) is 107°.
NH₃ (ammonia) is considered a weak base because it can accept protons (H⁺) from acids in aqueous solutions, forming ammonium ions (NH₄⁺).
Questions related to
On Question asked by student community
Could you please clarify the following examples, or you just want me to explain the formula?
Sal ammoniac is the common name for ammonium chloride which is a white crystalline solid used in laboratories and some chemical processes.
Chemical name ammonium chloride
Molecular formula NH4Cl
It consists of one ammonium
Correct Answer: clues
Solution : The most appropriate option is the third option.
Explanation:
Clues refer to hints, evidence, or information that helps in understanding or solving something. In the context of the passage, moons, asteroids, and comets provide crucial information or hints about the formation and evolution of the
Correct Answer: including
Solution : The most appropriate option is the fourth option.
Explanation: Including means to contain as part of a whole. In this context, it implies that among the eight planets in the solar system, Earth is one of them. "The eight planets in the solar system, including
Correct Answer: 14:3
Solution : The correct answer is 14:3.
The mass ratio of nitrogen to hydrogen is 14:3. As a result, three hydrogen atoms and one nitrogen atom combine to form a molecule with a molecular weight of 17. Ammonia, or NH₃, is the formula of the compound
Correct Answer: orbit
Solution : The most appropriate option is the second option.
Explanation:
Orbit is the correct word to use in this context, as it refers to the motion of celestial objects around a central body due to gravitational pull. In the context of the solar system, planets, moons,
