Anions and Cations Difference - Meaning, Example, Types, Uses, FAQs
Atoms and electrons that gain and lose one valance Electron form the ions. The ion form after the gain of the electron is called an anion and the ion which forms after the loss of an electron is called a cation. The anion carries the negative charge and the cation carries the positive charge. A cation is smaller in size than an anion
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is a Cation?
- What is an Anion?
- Cation vs Anion
- Difference Between Cations and Anions:
- Uses of Cations and Anions:
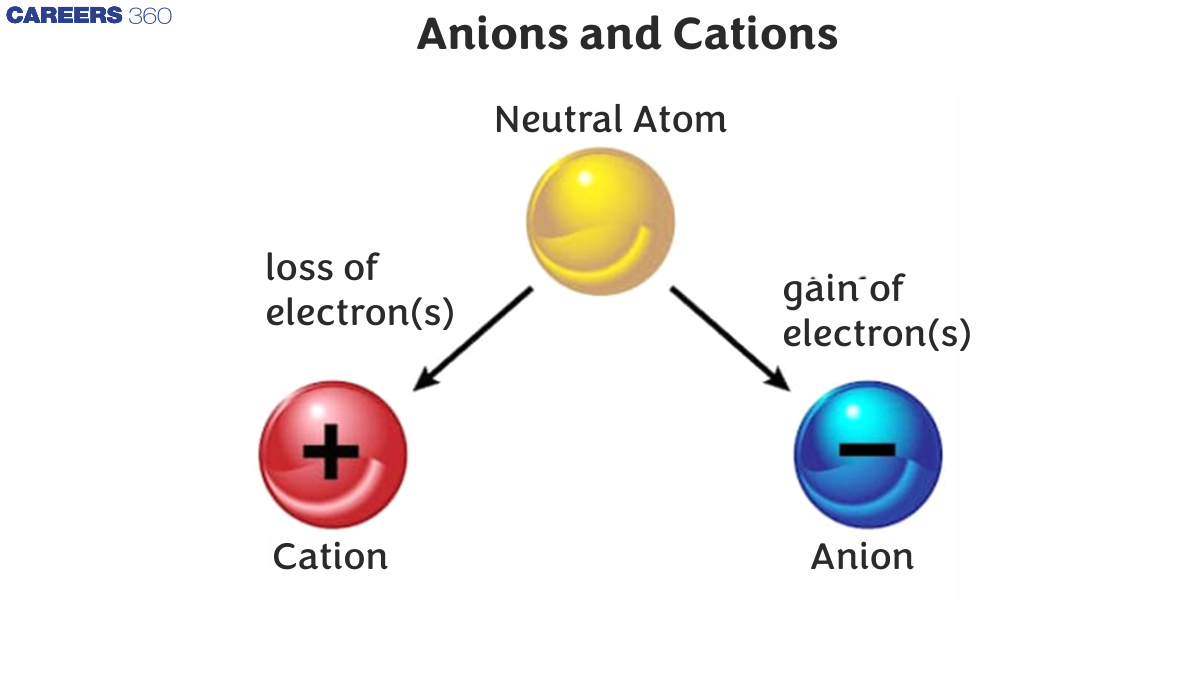
What Do you mean by an ION?
What is an Ion? Ions are a type of entity that is studied in science. Chemistry is made up of atoms and electrons that have gained or lost weight as a result of the removal or addition of one or more valence electrons, resulting in a positive or negative charge. Negative charge-bearing ions are known as anions, whereas positive charge-bearing ions are known as cations. Due to the fact that they both have charges of opposing characteristics, they are attracted to one another and create an ionic connection.
The word ion comes from the Greek word ἰόv,, which means "to travel." Ions are electrically charged particles. An ion is an atom or molecule with a positive Electric Charge. Because the number of electrons and protons in an atom is equal, they have no charge and are neutral in nature. While the amount of protons in ions is identical, the number of electrons is unequal. As a result, they are either positive or negative ions.
Examples of ions include Na+, O-2, NH4+, OH-, and others.
Polyatomic ions are a group of elements or atoms that are covalently connected but have either a positive or negative charge. Polyatomic ions include CN-, OH-, NH4+, NO3-, CO3-2, and others. Monoatomic ions are ions that have only one element and have either a positive or negative charge. Examine Na+, O-2, Al+3, Ce+3, and so on.
Related Topics
What is a Cation?
Cation meaning: The ions with a positive charge are referred to as cations. Examples of Cations include Na+, Al+3, Ce+3, and so on. When an atom loses an electron, it obtains a positive charge because its nucleus has fewer electrons than protons. The positively charged species is then referred to as a cation. Faraday and Whewell coined the phrase. It is derived from the Greek words (káto) ἰόv,, and (kation), both of which indicate “going down”.
Also, check-
What is an Anion?
Anion meaning: The ions with a negative charge are known as anions. Examples of Anions include O-2, CN-, OH-, Cl-, and others. When an atom gains electrons to achieve stability, it acquires a negative charge because its nucleus contains more electrons than protons. This negatively charged species is therefore referred to as an anion charge. Faraday and Whewell coined the phrase. It comes from the Greek word ἄvω ἰόv (anion) meaning “going up” Positive ions are called anions.
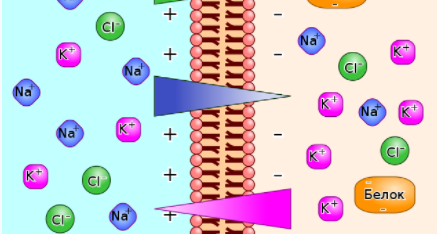
The given figure represents the membrane potential of ions Na+ and K+ represents the positively charged cations while Cl- represents the negatively charged anion.
Cation vs Anion
Positively charged ions are known as cations. Negatively charged ions are known as anions. Ions are positively or negatively charged atoms or molecules. A balanced atom will become a positively charged cation if one or more electrons are lost.
How to use Cation vs Anion-Remember that cations are positive ions, meaning they have lost one or more electrons and hence have more protons than electrons. Anions are negatively charged ions because they have acquired one or more electrons and so have more electrons than protons(cation vs anion).
Difference Between Cations and Anions:
The Major Difference Between an Anion and a Cation in Terms of Charge, Nature of the Element, and the Electrode are given as:
Basis | Anions | Cations |
Definition | An anion is an atom or molecule that is negatively charged. | A cation may be defined as an atom or molecule that is positively charged. |
Charge Type | Negative | Positive |
Type of Element | Non-Metal | Metal |
Type of Electrode used | Anode | Cathode |
Examples | Sulfide, Oxide, Fluoride, Chloride | Iron, Lead, Sodium |
The above table shows the difference between cation and anion(cation vs anion).
Related Topics |
Anions and Cation can be Distinguished from one Another as:
S.No | CATION | ANION |
A cation is an ion or a charged particle with a positive charge | An anion is an ion or a charged particle with a negative charge. | |
The word cation comes from the Greek word (káto) (kation), which means "to go down." | The word anion comes from the Greek word ἰόv (anion), which means "to rise." | |
The number of protons in cations is greater than the number of electrons | The number of electrons in anions is greater than the number of protons. | |
Metals, in general, create cations. | Non-metals, in general, form anions. | |
In electrolysis, cations are drawn to the negatively charged electrode. | In electrolysis, anions are drawn to the positively charged electrode. | |
Anions are larger than cations, whereas cations are smaller. | Anions are often larger than cations in size. | |
Cations gain electrons and become neutral atoms or molecules as a result. | Anions lose their electrons and become neutral atoms or molecules. | |
Ionic compounds are formed when cations and anions create electrostatic or ionic connections | Ionic compounds are formed when anions create electrostatic or ionic connections with cations | |
Cations include Na+, Mg+2, Ca+2, Fe+3, and so on. | Anions include O-2, Cl-, Br-, and others. |
Uses of Cations and Anions:
In our daily lives, cations play a crucial function. Blood pressure regulation and muscular contraction are both dependent on sodium, potassium, and magnesium ions. Calcium ions are a crucial component of bone structure. Water softeners can use sodium ions to eliminate other hazardous elements.
Anions are negatively charged ions that are generated when electrons outnumber protons in atoms or molecules. Anions and cations frequently interact to form salts, which are essential in the human body. From Hormones production to DNA construction, these particles are involved in a variety of important biological activities.
Also read -
| NCERT Solutions for Class 11 Chemistry | NCERT Notes Class 11 Chemistry |
| NCERT Solutions for Class 12 Chemistry | NCERT Notes Class 12 Chemistry |
| NCERT Solutions for All Subjects | NCERT Notes For All Subjects |
Frequently Asked Questions (FAQs)
The ions with a positive charge are referred to as cations. Cation example include Na+, Al+3, Ce+3 and so on. When an atom loses an electron, it obtains a positive charge because its nucleus has fewer electrons than protons. The positively charged species is then referred to as a cation.
Anions are larger than cations, whereas cations are smaller.
Examples of Cations include Na+,Al+3, Ce
Examples of Anions include O-2, CN-, OH-, Cl-
The word anion comes from the Greek word ἰόv (anion), which meaning "to rise." Also anion is negative.
Ions are electrically charged particles. Negative charge bearing ions are known as anions, whereas positive charge bearing ions are known as cations.
The primary difference lies in their charge: anions are negatively charged, while cations are positively charged. This difference affects how they interact with other ions and molecules.
Anions form when atoms or molecules gain electrons, which can happen through chemical reactions or when atoms attract electrons more strongly due to their electronegativity. Cations form when atoms lose electrons, often due to ionization during reactions or the influence of strong electronegative elements.
Also Read
06 Feb'25 11:21 PM
30 Dec'24 03:07 PM
20 Dec'24 11:57 PM
16 Dec'24 11:39 PM
16 Dec'24 11:27 PM
16 Dec'24 11:16 PM
21 Oct'24 04:01 PM
21 Oct'24 12:25 PM