Aromatic Compounds - Definition, Examples, Features, Characteristics, FAQs
Chemical compounds that consist of ring systems associated with pi-electrons separated by alternating double and single bonds. Aromatics requires Huckel's satisfying law. In the aromatic compound, there is the delocalization of pi-electrons in place of alternating double bonds. Benzene is a common example of an aromatic compound. This is an important topic of Organic Chemistry
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Aromatic Compounds Examples
- Features of Aromatic Chemicals
- Separation of Aromatic chemicals
- 1. Chemicals Incorporated into Nuclear Buildings
- 2. A series of localized chemicals
- Non-benzenoid Aromatic compounds
- IUPAC Appointment of Aromatic Chemicals
- The naming of IUPAC for hydrocarbons is described below:
- Reactivation of Aromatic Compounds
- Characteristics of aromatic compounds include:
- Heterocyclic compounds examples
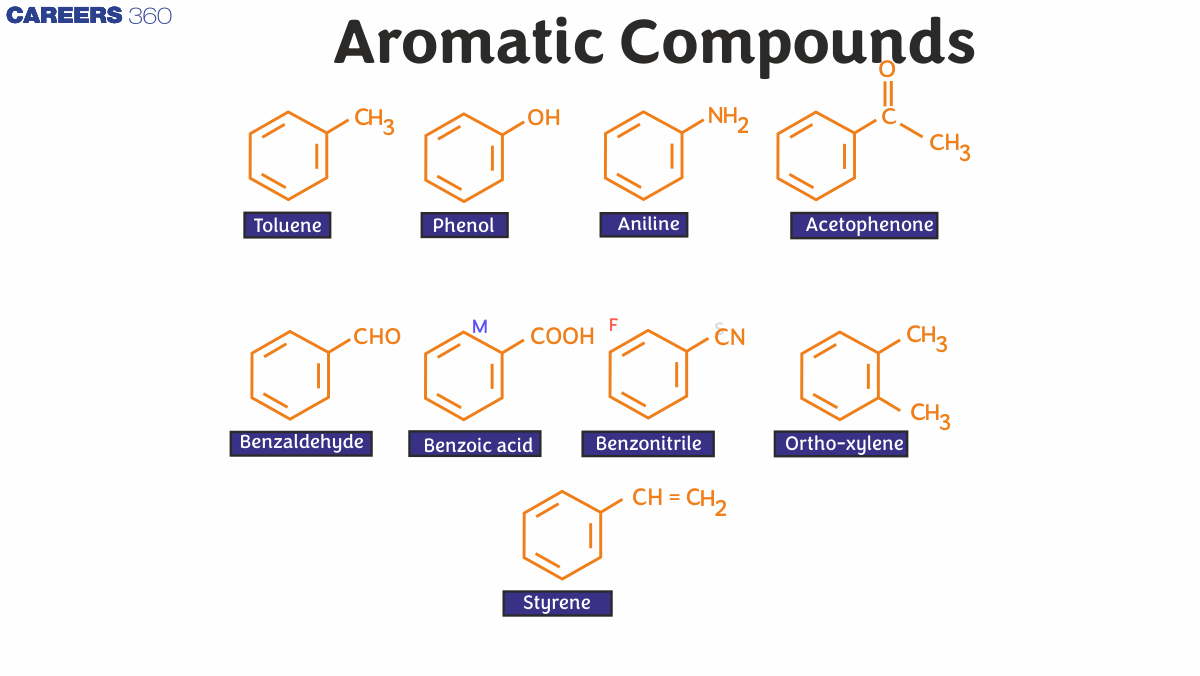
Aromatic Compounds Examples
Fragrant hydrocarbons, with hydrocarbons containing sigma bonds and pi electrons separated between carbon atoms in the ring.
For example, benzene. They are known as aromatic because of their pleasant aroma.
Any hydrocarbon can be classified as an aromatic compound as long as it follows the Huckel rule. According to Huckel's law, for a ring to smell, it must have the following properties: The planet Complete the electron renewal in the ring
Presence of (4n + 2) π electrons in the ring when n is the sum (n = 0, 1, 2, ..)
Huckel's Law of Aromaticity
Huckel's law states that only planets, fully monocyclic planets, have 4n + 2 electrons, where n is a whole number, i.e., n = 0, 1, 2, 3, 4, etc., which must have fragrant stability.
The fragrant compound must be planetary and contain a circular cloud of electrons below and above the plane of the molecule.
It has sp2 atoms separated and must comply with Huckel's law.
According to this rule, the ring system must have electrons (4n + 2) π electrones,
where n is a whole number (0, 1, 2, 3, etc.). At this time the ring systems have 2 (n = 0), 6 (n = 1), 10 (n = 2), 14 (n = 3) etc pi electrons.
Typical examples of aromatic chemicals are benzene, naphthalene, and anthracene.
Also check-
Features of Aromatic Chemicals
Arenes are mostly inactive and do nothing on the water. These compounds are generally ineffective and are used as solvents for other non-polar compounds. Their carbon and hydrogen ratios are high and therefore, they are characterized by a bright yellow flame.
Separation of Aromatic chemicals
The division of the arenes is based on the location of the active group.
They are divided into two and discussed below:
1. Chemicals Incorporated into Nuclear Buildings
In any fragrant object whenever any group absorbs or operates, it is directly connected to the benzene ring, known as a nuclear substitute.
2. A series of localized chemicals
In any fragrant object if the active group is present in the ring side series it is known as the compound chain substitute.
These compounds are called phenyl compounds of related aliphatic compounds.
Non-benzenoid Aromatic compounds
Azulene, which consists of a structure of seven-limbed and five-limbed rings, is a typical non-benzenoid fragrant compound.
Three effective methods of production have been identified so far as follows: The first method, developed by Ziegler and Hafner, is a ring-salt or pyrylium salt reaction with cyclopentadienides.
The second, developed by Nzoe and Seto et al. regenerative reactions of tropone containing halogen, methoxy, or tosyloxy groups in area 2 and active methylene compounds, such as cyanoacetates and malonates, where the base is located.
The latter method, developed by Yasunami and Takase et al., Reactions of oxaazulanones with enzymes.
The elements of tropolone are also classified as unhealthy benzenoid chemicals. Hinokitiol, the generic site of tropolone, is known to show an anti-bacterial effect.
And colchicine, an alkaloid compound with a tropone ring, shows strong antitumor effects.
Therefore, tropolone compounds have potential in the field of medicine, especially in anti-cancer drugs.
Related Topics
IUPAC Appointment of Aromatic Chemicals
Previously, most chemicals with the same structure were known by different names depending on the regions in which they were synthesized. This naming system was relatively simple because it caused a great deal of confusion. Finally, a standard naming system that registered general rules was developed by IUPAC (International Union for Pure and Applied Chemistry) for compounds.
This method of naming is the IUPAC or IUPAC surname.
The naming of IUPAC for hydrocarbons is described below:
1. According to the IUPAC sub-section of fragrant chemical substitutes, the concrete name is the starting point in the name of perfumed compounds. For example, a benzene ring attached to a single nitro group is called a nitrobenzene.
2. When more than one identical group is in the ring, they are labeled with Greek numerical prefixes such as di, tri, tetra to indicate the number of identical groups included in the ring. When two groups of bromo are attached to carbon atoms near the benzene ring, it is called 1,2-dibromobenzene.
3. When different replacement groups are attached to fragrant chemicals, the base component is given the first number and the numerical method is selected in such a way that the next substitute gets the lowest number. The elements are called alphabets.
4. In the case of fragrant chemicals substituted for many others, sometimes words such as ortho (o), meta (m) and para (p) are also used as prefixes to indicate positions related to 1,2-; 1,3- and 1,4- respectively.
5. When an alkane and a functional group are attached to a fragrant mixture, the fragrant mixture is considered the position, rather than the parent. For example: when a benzene ring is attached to an alkane by the active group, it is considered a phenyl bond, defined by Ph-.
The physical properties of fragrant chemicals
Fragrant compounds are usually non-polar and waterproof. Since they are usually ineffective, they are just as effective as solvents for other non-polar chemicals. Because of its high carbon and hydrogen content, aromatic compounds are characterized by a bright yellow flame.
Also read -
| NCERT Solutions for Class 11 Chemistry | |
| NCERT Solutions for Class 12 Chemistry | |
| NCERT Solutions for All Subjects |
Reactivation of Aromatic Compounds
Double bonds to aromatic chemicals are less likely to contribute to further reactions than those found in conventional alkenes.
Instead, cyclic aromatic compounds undergo electrophilic substitution changes. This stability is lost with the addition of electrophilic because the product is odorless.
Characteristics of aromatic compounds include:
1.It has to be Cyclic.
2.You must have (4n + 2) pi Electrons (n = 1,2,3,4, ...)
3.Oppose Add but Select Substitutions.
4You Must Find the Power of Resonance.
Related Topics
What does it mean when something smells aromatic ring?
A scented molecule or compound is one that has special properties and properties due to the closed loop of electrons. Fragrant molecules are sometimes simply called aromatic rings.
Fragrant molecules are called aliphatic. If a molecule contains a fragrant sub-unit, this is often called an aryl group.
What is an aromatic combination in simple words?
The fragrant computer is any large class of incomplete chemicals characterized by one or more atoms of atoms joining the bonds of two different types.
What Is the main source of perfumes?
Coal and gasoline.
Coal is a complex mixture of a large number of chemicals, most of which are long chemical cables. When the coal burns up to 1000 ° C in the absence of oxygen, the flexible components, called bitumen oil, are released.
What smells aromatic?
Chemicals that contain one or more separate or mixed benzene rings are called fragrant chemicals. Where the orbital / electron binding is not limited to only two atoms but spreads over more than two atoms at a time, such orbital / electrons are called delocalized and this process is known as delocalization.
Heterocyclic compounds examples
A heterocyclic compound, also called a heterocycle, is a large chemical compound that is characterized by some or all of the atoms in their molecules attached to rings that contain at least one element atom other than carbon (C).
The circular part (from the Greek cyclos, meaning “circle”) of the heterocyclic indicates that at least one ring structure exists in that compound, whereas the prefix hetero- (from Greek heteros, meaning “other” or “different”) refers to carbon-free atoms, or heteroatoms, in the ring.
By their design, heterocyclic aromatic compounds examples are similar to cyclic organic compounds that only incorporate carbon atoms into rings - for example, cyclopropane (with a three-carbon atom ring) or benzene (with a six-carbon atom ring) —but the presence of Heteroatoms provides heterocyclic compounds examples in the body and chemicals that are often very different from those of all-carbon-ring analogs.
According to the orbital molecular structure of the benzene structure, the benzene ring incorporates the formation of three orbitals divided by all six atoms in carbon, while the valence bond theory defines two stable ring structures.
Frequently Asked Questions (FAQs)
Aromaticity is a substance in cyclic organic chemistry, a planar (flat) with resonance bond rings that provide greater stability compared to other geometric or contact arrangements with the same set of atoms.
A heterocyclic compound is a living compound where a non-carbon atom incorporates one of the carbon atoms into the molecule. Nitrogen, oxygen, and sulfur are common hetero atoms.
Fragrant hydrocarbons are compounds that contain benzene as part of their structure, also known as aromatic chemicals. Benzene, with the formula C6H6, is a circular hydrocarbon.
Carbon compounds are directly related to the chemical composition of aliphatic compounds. In aromatic chemicals, Carbon compounds are associated with pi electrons that are bonded in the form of a ring structure.
Common examples of aromatic compounds include:
- Benzene (C6H6)
- Toluene (C7H8)
- Naphthalene (C10H8)
- Aniline (C6H5NH2)
- Phenol (C6H5OH)
Aromatic compounds can be synthesized through several methods, including:
- Electrophilic Aromatic Substitution (EAS): A common reaction where an electrophile replaces a hydrogen atom in the aromatic ring.
- Friedel-Crafts Reaction: A type of EAS involving alkylation or acylation of an aromatic ring with alkyl halides or acyl halides.
- Cyclization reactions: Such as the preparation of aromatic rings from non-aromatic precursors.
Also Read
12 Dec'24 04:49 PM
04 Nov'24 05:31 PM
21 Oct'24 05:53 PM
09 Oct'24 06:12 PM
09 Oct'24 04:00 PM
30 Sep'24 10:57 AM
30 Sep'24 08:52 AM

