Atomic Mass of Elements - Overview, Examples, Comparison, FAQs
The atomic mass of an element can be measured by the unification of atomic mass units. Considering that a carbon-12 atom is at rest, one unified unit of atomic mass equates to one-twelfth of its mass. The atomic mass of a given element is almost equal to its mass number since protons and neutrons are responsible for the majority of its mass.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Why do elements have different atomic masses?
- The atomic mass of first 20 elements to 30 elements
- Atomic mass of an element compared with its atomic number
The atomic mass of an element can be used in chemistry if the atomic mass of an element is combined with the idea of a mole: one mole of an element has a mass in grams equal to its atomic mass of elements in amu. One mole of iron atoms weighs 55.847 grams due to its atomic mass of elements of 55.847 amu. Ionic compounds and molecules can be treated in the same way.
Why do elements have different atomic masses?
Elements are described by their atomic mass. An atom's mass is the combined mass of all its protons. The unit is known as the unified atomic mass unit and is represented by 'u'.
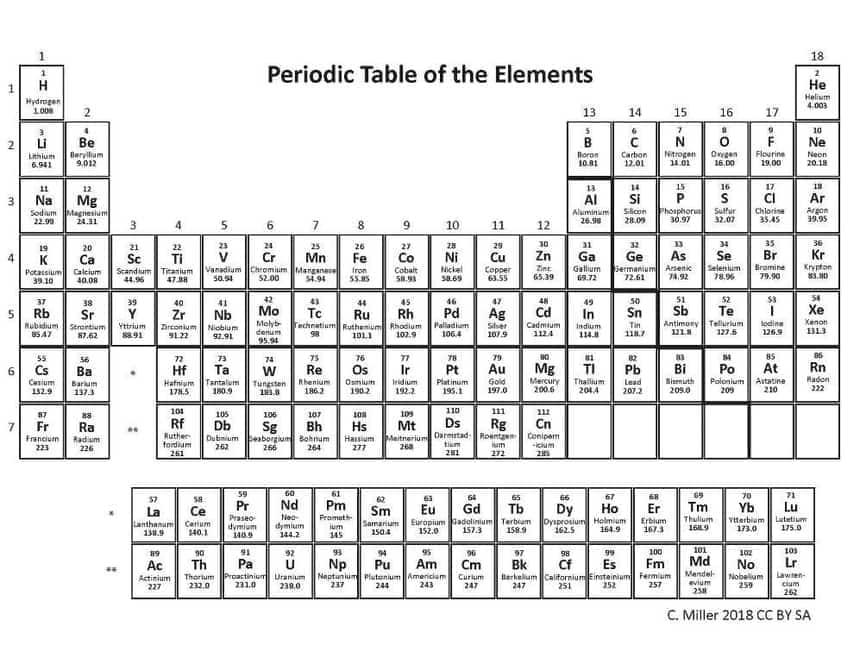
As a measure of the average atomic mass of a mixture of isotopes in a given sample of an element, standard atomic atomic weight is used. Each element has different set of protons and neutrons in its nucleus, hence different atomic masses are observed. Below is the modern periodic table of elements depicting atomic number and atomic mass of the elements.
Apart from atomic mass, trends for various other physical quantities such as ionisation enthalpy, electronegativity etc. can be analysed from the modern periodic table.
Also read -
The atomic mass of first 20 elements to 30 elements
The following table contains a list of the atomic mass of first 30 elements ordered by atomic number and atomic mass.
Atomic Number | Element | Atomic Mass |
1 | Atomic Mass of Hydrogen | 1.008 |
2 | Atomic Mass of Helium | 4.0026 |
3 | Lithium Atomic Mass | 6.94 |
4 | Atomic Mass of Beryllium | 9.0122 |
5 | Boron Atomic Mass | 10.81 |
6 | Atomic Mass of Carbon | 12.011 |
7 | Atomic Mass of Nitrogen | 14.007 |
8 | Atomic Mass of Oxygen | 15.999 |
9 | Atomic Mass of Fluorine | 18.998 |
10 | Atomic Mass of Neon | 20.180 |
11 | Atomic Mass of Sodium | 22.990 |
12 | Atomic Mass of Magnesium | 24.305 |
13 | Atomic Mass of Aluminium | 26.982 |
14 | Atomic Mass of Silicon | 28.085 |
15 | Atomic Mass of Phosphorus | 30.974 |
16 | Atomic Mass of Sulphur | 32.06 |
17 | Atomic Mass of Chlorine | 35.45 |
18 | Atomic Mass of Argon | 39.948 |
19 | Atomic Mass of Potassium | 39.098 |
20 | Atomic Mass of Calcium | 40.078 |
21 | Atomic Mass of Scandium | 44.956 |
22 | Atomic Mass of Titanium | 47.867 |
23 | Atomic Mass of Vanadium | 50.942 |
24 | Atomic Mass of Chromium | 51.996 |
25 | Atomic Mass of Manganese | 54.938 |
26 | Atomic Mass of Iron | 55.845 |
27 | Atomic Mass of Cobalt | 58.933 |
28 | Atomic Mass of Nickel | 58.693 |
29 | Atomic Mass of Copper | 63.546 |
30 | Atomic Mass of Zinc | 65.38 |
The above table shows the atomic mass of all elements or atomic number of elements from 1 to 30. Such details can help in knowing the various physical quantities of elements.
To calculate a molecule's molecular mass, add the mass of each of its constituent atoms. There are several methods of finding the atomic mass of an element, but the simplest is to look it up on the periodic table.
Atomic mass of an element compared with its atomic number
Learn how elements differ from one another in terms of their atomic number and atomic mass now.
Atomic mass | Atomic Number |
An element's atomic mass is proportional to the number of neutrons and protons that make up its nucleus. | Essentially, the nuclear number is the number of protons within the nucleus of a given element. |
A particular element's average atomic weight is referred to as its average. | The number of nucleons present in an atom's nucleus is the number of nucleons in total. |
The letter A is used for representing the atomic mass. | Atomic number Z is represented by the letter Z. |
Element types cannot be determined by atomic mass. | A chemical element can usually be classified and identified by its atomic number. |
Different isotopes of an element are classified according to their atomic masses | An element's atomic number is the same only for its isotopes. |
The atomic mass unit (AMU) is always used to measure atomic mass. | For elements to be placed in a periodic table, the atomic number is simply a digit. |
Frequently Asked Questions (FAQs)
Atomic mass is the mass of an atom, typically expressed in atomic mass units (amu), and it represents the average mass of all isotopes of an element, weighted by their natural abundance.
Atomic mass is calculated by taking the weighted average of the masses of all naturally occurring isotopes of an element, based on their relative abundance.
Atomic mass is not a whole number because it is an average of the masses of different isotopes, which have slightly different masses, and the natural abundances of these isotopes vary.
Atomic mass refers to the mass of an atom, while atomic number is the number of protons in the nucleus of an atom, determining its element identity.
The element with the highest atomic mass is Oganesson (Og), with an atomic mass of around 294 amu.
Also Read
12 Mar'25 09:34 AM
06 Feb'25 11:45 PM
06 Feb'25 11:36 PM
09 Jan'25 03:51 PM
16 Dec'24 11:23 PM
13 Nov'24 03:53 PM
23 Sep'24 01:11 PM