Atomic Number Mass Number - Definition, Example, Formula & Calculation, FAQs
Atomic number of an element can be defined as the number of protons present in the nucleus which can be denoted by the symbol Z, and simply as we can better understand the atomic number by taking an example i.e. if we see the hydrogen atom which contains one proton so the atomic number of hydrogen atom will be one, similarly for the sodium atom it will be 11 owing to the total 11 proton in its nucleus.
- Atomic mass
- Atomic number and atomic mass of elements
- Calculation of Atomic Number and Mass Number:
- More Summary About Atomic number and Mass Number:
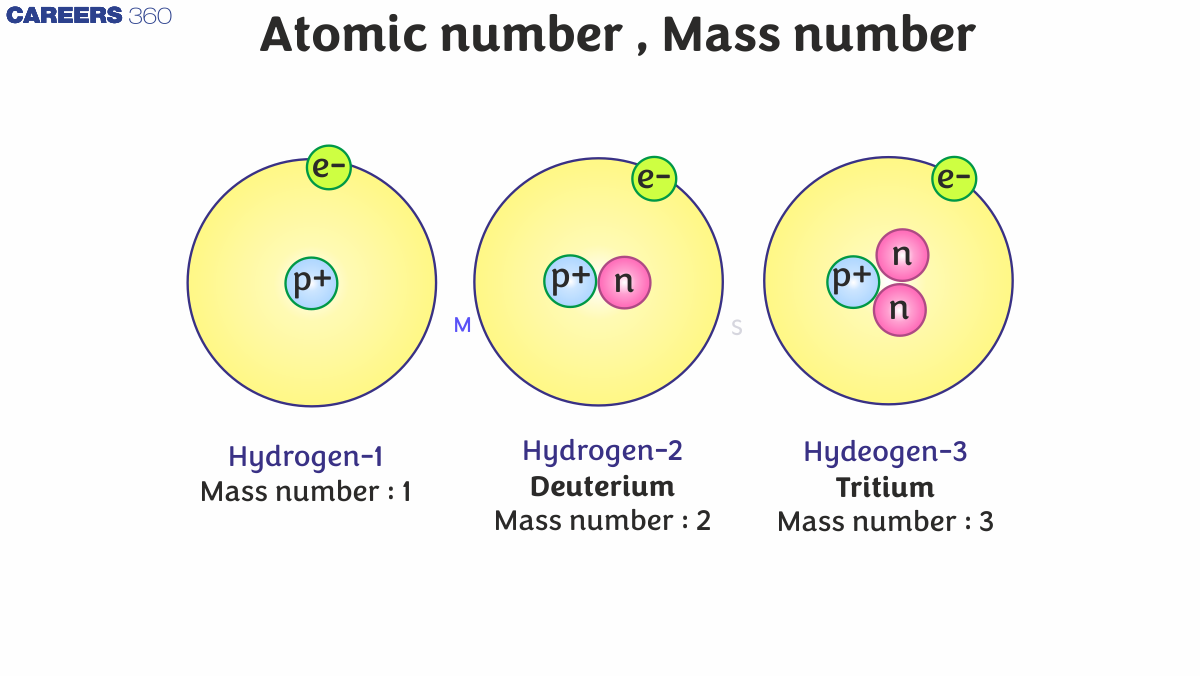
So, from the above fact we can say that the dependency of the atomic number is based only on the protons in the element not on the electrons or neutrons, so by using this statement we can create a relation mathematically as;
Z = no. of protons = no. of electrons
Below is the complete representation of an elements with its symbol, atomic number and mass number.
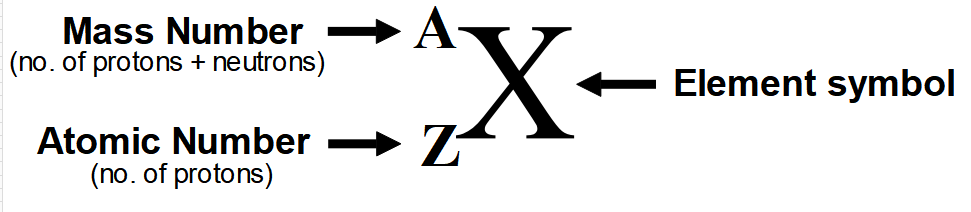
This way, we can represent atomic mass and atomic number of any element.
Atomic mass
Atomic mass of an element is the sum of protons and neutron in the nucleus and is denoted b the symbol "A" as shown in the above image, so mathematically we can write as:
A = Protons + Neutrons
This combination of protons and neutrons is also called nucleons.
We can also write.
A = Z + Neutrons
Neutrons = A – Z
In the periodic table, the hydrogen element is only the element which does not have the neutron and has only one proton.
Atomic number and atomic mass of elements
The atomic mass of an element is actually very small because atoms are extremely small. Today we have sophisticated techniques i.e., mass spectroscopy for determining the atomic masses fairly and accurately. But in the 19th century scientists could determine the mass of an atom relative to another by experimental means as has been mentioned earlier.
Name of Elements | Symbol | Atomic number | Number of Electrons | Number of Protons | Number of neutrons | Atomic Mass |
Hydrogen | H | 1 | 1 | 1 | - | 1 |
Helium | He | 2 | 2 | 2 | 2 | 4 |
Lithium | Li | 3 | 3 | 3 | 4 | 7 |
Beryllium | Be | 4 | 4 | 4 | 5 | 9 |
Boron | B | 5 | 5 | 5 | 6 | 11 |
Carbon | C | 6 | 6 | 6 | 6 | 12 |
Nitrogen | N | 7 | 7 | 7 | 7 | 14 |
Oxygen | O | 8 | 8 | 8 | 8 | 16 |
Fluorine | F | 9 | 9 | 9 | 10 | 19 |
Neon | Ne | 10 | 10 | 10 | 10 | 20 |
Sodium | Na | 11 | 11 | 11 | 12 | 23 |
Magnesium | Mg | 12 | 12 | 12 | 12 | 24 |
Aluminium | Al | 13 | 13 | 13 | 14 | 27 |
Silicon | Si | 14 | 14 | 14 | 14 | 28 |
Phosphorus | P | 15 | 15 | 15 | 16 | 31 |
Sulphur | S | 16 | 16 | 16 | 16 | 32 |
Chlorine | Cl | 17 | 17 | 17 | 18 | 35.5 |
Argon | Ar | 18 | 18 | 18 | 22 | 40 |
Potassium | K | 19 | 19 | 19 | 20 | 39 |
Calcium | Ca | 20 | 20 | 20 | 20 | 40 |
The above table contains the element name, atomic number, atomic mass, number of protons, number of electrons, and number of neutrons, so we can easily differentiate between them, in inorganic chemistry we can also find such type of data and we can clearly learn the increasing or decreasing number of atom or mass by going from up to down the periodic table. An element’s number is adequate to the number of protons in the nuclei of any of its atoms. The table of elements in periodic table gives the atomic number of every element. The number may be a integer usually written above the chemical symbol of every element within the table. The number for hydrogen is 1 because every hydrogen atom has proton.
The number for helium is because every helium atom has 2 protons, the number of elements within the table, that are arranged as of accelerating number of protons within the nucleus. Accordingly, the protons, which is usually adequate to the number of electrons within the neutral atom, is additionally the number, for a while let’s say iron has 26 no of electron it means it has its atomic number 26. As we have discussed above the number of an element is the sum of proton and neutron now, we will discuss on the calculation the atomic number and mass no.
Also check-
Calculation of Atomic Number and Mass Number:
Our great scientists calculated the mass of atom by the mean of mass number from the isotopes of the elements that occurs naturally. Often the decimal number is obtained in the results. For instance, the atomic mass of chlorine is 35.45amu due to its various isotopes.
Let us understand with an example say an atomic number (Z) and mass number (A), in a neutral atom we can find the number of protons, neutrons and electrons, for instance a lithium atom (Z=3, A=7 amu) contains three protons (from Z), three electrons (as proton = electron), and four neutrons (7 – 3 = 4).
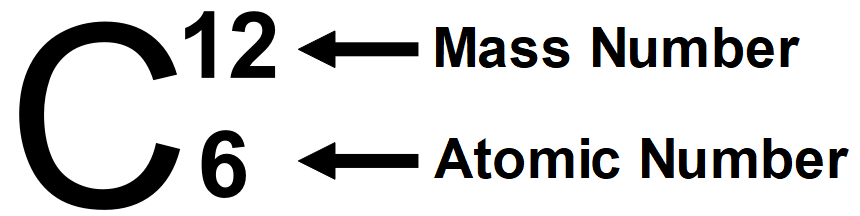
More Summary About Atomic number and Mass Number:
It is well known that protons are present within the nucleus of an atom. atomic number of any element is determined by the amount of proton present in atom, it's denoted by ‘Z’. All atoms of a component have an equivalent number, Z. In fact, elements are defined by the amount of protons they possess. For hydrogen, Z = 1, because in atom, just one proton is present within the nucleus. Similarly, for carbon, Z = 6. Therefore, the number is defined because the total number of protons present within the nucleus of an atom.
By studying the properties of the subatomic particles of an atom, we can easily say that the total mass of an atom is practically thanks to protons and neutrons alone. that's by protons and neutrons also are called as nucleons. So therefore, the total mass of an atom resides in its own nucleus. for instance, mass number of carbons is 12amu due to its 6 protons and 6 neutrons. In nature, variety of atoms of some elements are identified, which have the same number of atom but different mass numbers which are called the isotopes for example hydrogen element has its isotopes named deuterium and tritium denoted by simply D and T.
Let us consider two elements calcium (Ca), atomic number 20, and argon (Ar), atomic number 18. the number of electrons in these atoms is different, but the nucleon number of both these elements is 40.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Frequently Asked Questions (FAQs)
The atomic number can be defined as the protons present in the nucleus which can be denoted by the symbol Z.
Atomic mass of an element is the sum of protons and neutron in the nucleus and is denoted by the symbol A and mathematically it can be written as
A = Protons + Neutrons
| ATOMIC NUMBER | ELEMENT | ATOMIC MASS |
|---|---|---|
| 1 | Hydrogen | 1.008 |
| 2 | Helium | 4.0026 |
| 3 | Lithium | 6.94 |
| 4 | Beryllium | 9.0122 |
Hydrogen is the element having atomic number =1.
Also Read
06 Feb'25 11:21 PM
30 Dec'24 03:07 PM
20 Dec'24 11:57 PM
16 Dec'24 11:39 PM
16 Dec'24 11:27 PM
16 Dec'24 11:16 PM
21 Oct'24 04:01 PM
21 Oct'24 12:25 PM

