Atomic Size & Atomic Radius
Atomic size or atomic radius is another basic quantity in chemistry that characterizes the dimensions of the atom. It is the true covalent bond length; it is equal to half of the distance between the nucleus of two successive atoms of the same element when are linked together by a covalent bond. As one traverses across the periodic table, atomic size conversely reduces and also increases from the top of the group to the bottom of the group. This trend depends on the value of the principal quantum number, n, and the number of effective nuclear charges felt by the outermost electrons. When electrons are grouped in the same energy level or shell, the orbitals successively get bigger and hence the size of the atom increases. In contrast to this, a higher value of nuclear charge makes electrons to be held closer to the nucleus thus reducing the atomic radii.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Scaling the Dimensions of Atoms: Exploring Atomic World
- Variation in a Period
- Atomic Properties
- Some Solved Example
- Conclusion
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
In this article, we will cover the concept of the Atomic radius, its size, and its variations in the periodic table. This concept falls under the broader category of Atomic structure, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE and more. Over the last ten years of the NEET and JEE exams (from 2013 to 2023), twelve questions have been asked on this concept, and twenty-five questions in JEE from 2015 to 2020 from this concept.
Scaling the Dimensions of Atoms: Exploring Atomic World
Various physical properties follow the trend according to the atomic numbers of elements.
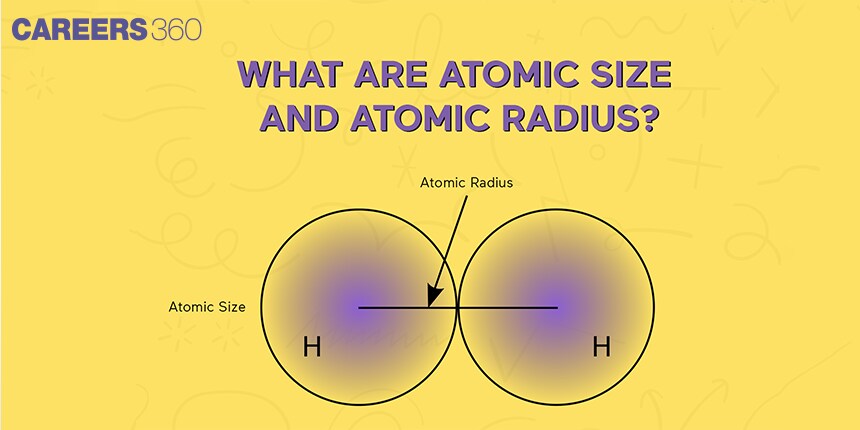
Atomic Radius
Atomic radius is the distance from the center of the nucleus to the outermost shell of electrons. It can be measured by x-ray diffraction, electron diffraction techniques, and other spectroscopic methods. As it cannot be measured in absolute form thus it is measured in various definitions such as covalent radius, Van der Waal’s radius, metallic radius, and ionic radius.Covalent Radius
It is half of the total length between two successive nuclei covalently bonded to each other in a molecule. Suppose there are two same atoms’ A’ and ‘A’ in a molecule and their bond length is ‘a’, then the covalent radius is half of the covalent bond length between A and A. Thus, covalent radius = (a/2).

- Metallic Radius
It is defined as half of the distance between nuclei of two adjacent metal atoms that are closely packed in the metallic crystal lattice. For example, if there are two metal atoms ‘A’ and ‘A’ that are closely packed to each other and the bond length is ‘a’ then the metallic radius is half of the distance between these two metallic atoms i.e., a/2.

- Van der Waal’s Radius
It is half of the distance between two nuclei of the adjacent non-bonded atoms of different molecules. For example, if ‘a’ is the distance between two adjacent atoms i.e., A and B, then Van der Waals’s radius is half of the distance between these two atoms A and B, i.e., a/2.

- Ionic Radius
It is the effective distance from the center of the nucleus of an ion up to which it influences the electrons.
Variation in a Period
In moving from left to right in a period, the nuclear charge increases and the last electron enters the same shell, thus the effective nuclear charge increases. Thus in this way, the atomic size decreases in the period.

Variation in a Group
In moving from top to bottom in a group, the number of shells increases due to which the atomic size increases.

The size of the cation is always smaller than its parent atom. In the case of cations, the number of electrons in the ion decreases and the nuclear charge remains the same, thus the effective nuclear charge increases and the size decreases.
The size of the cation decreases as the effective nuclear charge increases
M+3 < M+2 < M+ < M
The size of the anion is always greater than its parent atom. In the case of anions, the number of electrons in the ion increases and the nuclear charge remains the same, thus the effective nuclear charge decreases and the size increases.
M-3 > M-2 > M- > M
Atomic Properties
Atomic properties are the physical properties of elements that are related to the atomic number of the elements. These properties can be divided into two categories:
Properties of individual atoms: These are the properties of individual atoms that are directly dependent on their electronic configurations. Some examples include ionization enthalpy, electron gain enthalpy, screening effect, effective nuclear charge, etc.
Properties of the group of atoms: These are properties of the group of atoms together that are indirectly related to their electronic configurations. Some examples include the melting point, boiling point, the heat of fusion, density, etc.
The Screening effect or Shielding effect
The decrease in the force of attraction between the outer electrons and the nucleus due to the presence of inner electrons is called the screening effect or shielding effect. These inner electrons generate the repulsion between these inner electrons and the outer electrons due to which the net force of attraction between the nucleus and the outer electrons decreases.
Calculation of the screening effect
For ns or np orbital electrons
All electrons in the (ns, np) group contribute to 0.35 each to the screening effect constant. Except for 1s electrons which contribute by 0.30.
All electrons in the (n-1) shell contribute by 0.85 each to the screening effect constant.
All electrons in (n-2) shell or lower contribute by 1.0 each to the screening effect constant.
For d- or f-electrons
All electrons in the (ns, np) group contribute to 0.35 each to the screening effect constant.
All the electrons in groups lower than (nd, nf) contribute by 1.0 each to the screening effect.
Effective Nuclear Charge
Due to the screening effect of the inner or the same shell electrons, the net force of attraction between the nucleus and the outer electrons decreases. This decreased force of attraction is known as an effective nuclear charge. It is represented by Z*. Mathematically, it can be formulated as:
Z* = (Z- $\sigma$), where $\sigma$ is the screening effect constant.
- In a period, the effective nuclear charge increases in moving from left to right.
II Period | Li | Be | B | C | N | O | F | Ne |
Z | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
? | 1.7 | 2.05 | 2.40 | 2.75 | 3.10 | 3.45 | 3.80 | 4.15 |
Z* | 1.3 | 1.95 | 2.60 | 3.25 | 3.90 | 4.55 | 5.20 | 5.85 |
In a group, the effective nuclear charge almost remains the same.
Group I | Li | Na | K | Rb | Cs |
Z | 3 | 11 | 19 | 37 | 55 |
? | 1.7 | 8.8 | 16.8 | 34.8 | 52.8 |
Z* | 1.3 | 2.2 | 2.2 | 2.2 | 2.2 |
Isoelectronic species -
A series of atoms, ions, and molecules in which each species contains the same number of electrons but a different nuclear charge.
e.g. N3-,O2-,F-,Ne,Na+,Mg2+,Al3+
Some Solved Example
Example 1:The correct order of atomic radii is :
1) N > Ce > Eu > Ho
2) Ho > N > Eu > Ce
3) (correct) Eu > Ce > Ho > N
4) Ce > Eu > Ho > N
Solution: The atomic radius gradually decreases along with the series. If we consider the atomic (i.e., metallic) radii for the lanthanides, two peaks appear at (63Eu [Xe] 4f7 5d0 6s2) and 70Yb [Xe] 4f14 5d0 6s2.
Eu and Yb each can provide only 2 electrons for metallic bonding while the other members each can provide 3 electrons for the bonding purpose.
Thus,
Eu > Ce > Ho > N
Hence, the answer is the option (3).
Example 2: Among the following ionic radii, choose the correct option:
1) K+ > Cl-
2) Na < Na+
3) Cl > Cl-
4) (correct) P3+ > P5+
Solution: Variation of Atomic Radii and ionic radii -
Comparison of the ionic radii and atomic radii
Thus, the size of cation ∝ 1/Zeff
M+3 < M+2 < M+ < M
Thus, the size of anion ∝ 1/Zeff
M-3 > M-2 > M- > M
The size of a cation is always less than that of an atom, and the size of an anion is always greater than that of an atom. Again, a more positively charged cation is smaller in size than a less positively charged cation.
Hence, the answer is the option (4).
Example 3:Which one of the following ions has the highest value of ionic radius?
1) Li+
2) B3+
3) (correct) O2-
4) F-
Solution: The ionic radius is the distance between the nucleus of an ion and the point where the nucleus exerts its influence on the electron cloud.
rcation +ranoin =rionic radii
The radius of a cation is invariably smaller than that of the corresponding neutral atom.
Na(1s22s22p63s1),Na+(1s22s22p6)
The radius of an anion is invariably bigger than that of the corresponding atom.
Cl(1s22s22p63s23p5),Cl−(1s22s22p63s23p6)
As the ze ratio increases, thle size decreases, and vice versa.
For Li+,ze=32=1.5 For F-,ze=910=0.9 For O2-,ze=810=0.8 For B3+,ze=52=2.5
Hence, the answer is the option (3).
Example 4:The ionic radii are in order:
1)$\mathrm{F}^{-}>\mathrm{O}^{2-}>\mathrm{Na}^{+}>\mathrm{Mg}^{2+}$
2) (correct) $\mathrm{O}^{2-}>\mathrm{F}^{-}>\mathrm{Na}^{+}>\mathrm{Mg}^{2+}$
3) $\mathrm{Mg}^{2+}>\mathrm{Na}^{+}>\mathrm{F}^{-}>\mathrm{O}^{2-}$
4) $\mathrm{O}^{2-}>\mathrm{F}^{-}>\mathrm{Mg}^{2+}>\mathrm{Na}^{+}$
Solution: We know these about ions-
O-2F- Mg2+Na+
z 8 9 11 12
e- 10 10 10 10
$\frac{\mathrm{z}}{\mathrm{e}}$ 0.8 0.9 1.1 1.2
We know as the (z/e) ratio increases size decreases.
Thus correct ionic radii order is
$\mathrm{O}^{2-}>\mathrm{F}^{-}>\mathrm{Na}^{+}>\mathrm{Mg}^{2+}$Therefore, the correct option is (2).
Example 5: Which of the following orbital has the least screening power?
1) s-orbital
2) p-orbital
3) d-orbital
4) (correct) f-orbital
Solution: Comparison of screening power -
Due to the different shapes and orientations of different orbitals, the screening power decreases from s to f.
f-orbitals are fundamental orbitals that have diffused shapes and because of this, it has the least screening power.
Hence, the answer is the option (4).
Example 6: Which of the following facts is/are true for the variation of the shielding effect in the periodic table?
1) Increases as we move left to right in a period
2) Increases down the group
3) (correct) Both a & b
4) decreases down the group
Solution: The shielding effect is a phenomenon by which the attraction of the nucleus on valence electrons is reduced due to inner electrons' repulsions.
The greater the size of the atom greater be shielding effect.
The shielding effect is a screening effect.
In the periodic table, the shielding effect increases from top to bottom in a group Due to increases in the number of electrons in the inner shells
In the periodic table, this effect decreases from left to right in a period due to no change in the number of shells.
Example 7: Which of the following orders shows the correct decreasing order of effective nuclear charge?
1) F>N>O
2) O>N>F
3) N>O>F
4) (correct) F>O>N
Solution: Variable of effective nuclear charge in the period -
It is observed that the magnitude of effective nuclear charge increases in a period when we move from left to right.
So, N < O < F (left to right).
Hence, the answer is the option (4).
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Conclusion
Most chemistry entails utilizing atomic radius as a measurement, and it follows a clear pattern on the periodic table affecting the matter’s features. As to the trends in atomic radius, it will be found that down a group or column atomic radius increases as more shells are formed to make the atomic size bigger. Periodically, atomic slice increases across periods from left to right because of the increase in the number of effective nuclear charges, which attract the electrons closer to the nucleus. This affects chemical reactivity; the ability or readiness of an atom to lose electrons or the strength with which an atom attracts electrons determines the size of the atom. All these trends influence the variety of elements’ behaviors, from malleable metals with large atomic radii up to reactive non-metals with atomic sizes as small as possible, determining the variety of the chemical and material world.
Frequently Asked Questions (FAQs)
atomic radius is a measurement, and it follows a clear pattern on the periodic table affecting the matter’s features.
The atomic radius of an atom often decreases over time.
The atomic radius increases down the group.
The Greater the size of the atom greater be shielding effect.
Also Read
12 Mar'25 09:34 AM
06 Feb'25 11:45 PM
06 Feb'25 11:36 PM
09 Jan'25 03:51 PM
16 Dec'24 11:23 PM
13 Nov'24 03:53 PM
23 Sep'24 01:11 PM