Carboxylic Acids: Definition, Structure, Properties, Formula and Uses
Think of the sour taste of vinegar or the citrus odor of lemons; think of the relieving action of aspirin. Everyday things that share a common chemical platform are carboxylic acids. Since carboxylic acids form an inseparable part of everyday life, their properties, methods of preparation, and key reactions are very essential for any person approaching organic chemistry. These acids are very important not only in biological cycles but also in many broad-area industrial applications.
- How to Understand Carboxylic Acids
- Chemical Properties and Acidity
- Key Reactions
- Relevance and Applications
- Some Solved Examples
- Summary
By nature, carboxylic acids are rather versatile compounds due to their functional group—the carboxyl group $(-\mathrm{COOH})$ which imparts very peculiar chemical properties and reactivity. This usually allows a wide array of possible chemical reactions that carboxylic acids take part in, and it plays a very important role in the synthesis of a myriad of compounds. Different methods of preparation of Carboxylic Acids are of importance and have different applications.
How to Understand Carboxylic Acids
A carboxylic acid can be defined as an organic compound that contains a carboxyl group,$-\mathrm{COOH}$Such a functional group is one that consists of a carbonyl, C=O, and a hydroxyl group, OH, attached to one carbon atom. The general formula of the carboxylic acid is $\mathrm{R}-\mathrm{COOH}$ here, R is a hydrocarbon chain. These acids exhibit typical acidic behavior, which is a direct result of the possibility of the carboxyl group donating a proton in the solution. Their reactivity and utility in many organic synthetic reactions are direct consequences of their acidity. Their unique structure and reactivity make carboxylic acids the driving force of organic synthesis, therefore leading to a large number of compounds of central importance.
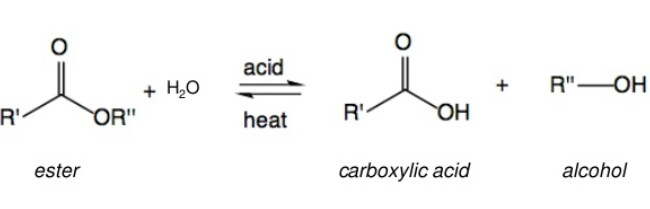
From Esters
Esters on acidic hydrolysis give acids, while on basic hydrolysis give carboxylates, which on acidification give corresponding acids. The reaction occurs as follows:

From Grignard reagents
Grignard reagents with dry ice form salts of carboxylic acids which after acidification give corresponding carboxylic acids. The reaction occurs as follows:
![]()
From Nitriles and Amides
![]()
Nitriles are hydrolyzed in an acidic or basic medium first to amides and then to acids. The reaction occurs as follows:

Chemical Properties and Acidity
There are several significant chemical properties of carboxylic acids. They do form hydrogen bonds and this has implications for their boiling and melting points. The acids are, by nature, typical acids since they neutralize with base forming salt plus water. Apart from that, carboxylic acids can undergo esterification with alcohol to yield esters and water. In addition, they can undergo reduction reactions to primary alcohols.
Formation of Anhydride
Carboxylic acid on heating with H2SO4 or P2O5 gives the corresponding anhydride. The reaction occurs as follows:

Reaction with Ammonia
On heating with NH3, carboxylic acid gives ammonium salt which on further heating at high temperature gives amides. The reaction occurs as follows:
![]()
Reduction
Carboxylic acids are reduced to$1^0$ alcohol by $\mathrm{LiAH}_4$ or better with $\mathrm{B}_2 \mathrm{H}_6 . \mathrm{B}_2 \mathrm{H}_6$ does not easily reduce esters, nitro, and halo groups, and $\mathrm{NaBH}_4$ does not reduce the (-COOH) group. The reaction occurs as follows:
![]()
Decarboxylation
When sodium salts of carboxylic acids are heated with soda lime$\left(\mathrm{NaOH}\right.$ and CaO in $3: 1$ ratio), they form hydrocarbons by losing $\mathrm{CO}_2$. This is known as decarboxylation. The reaction occurs as follows:![]()
Kolbe's electrolysis
The electrolysis of aqueous solution of sodium or potassium salts of carboxylic acid makes the carboxylic acid undergo decarboxylation to form alkane. The reaction occurs as follows:
Halogenation
The method of preparing α-chloro or α-bromo acid is by Hell-Volhard-Zelinsky reaction, which is carried out by treating the acid with $\mathrm{Cl}_2$ or $\mathrm{Br}_2$ in the presence of a small amount of red phosphorous. The reaction occurs as follows:
Ring substitution
Aromatic carboxylic acid undergoes substitution electrophilic reactions in which the $(-\mathrm{COOH})$ group acts as a deactivating and meta-directing group. It does not undergo Friedel-Crafts reaction because the $(-\mathrm{COOH})$ group is deactivating and the catalyst $\mathrm{AlCl}_3$gets bonded to the (COOH) group. The reactions occur as follows:


Acidity in Carboxylic Acids
One of the more important characteristics of carboxylic acids is their acidity. This is ordered in relation to the stability of the carboxylate anion, R-COO-, upon deprotonation. Electron-withdrawing groups onto the hydrocarbon chain, R, in such a structure improves acidity by stabilizing the carboxylate anion, and electron-donating groups diminish acidity. An excellent example is formic acid,1. $\mathrm{H}-\mathrm{COOH}$, that is more acidic relative to acetic acid, $\mathrm{CH}_3-\mathrm{COOH}$ since the attached methyl group in acetic acid is electron-donating.
Carboxylic acids are weaker than mineral acids but they are stronger than alcohols, phenols, and peroxy acids.
- Phenols are stronger acids than alcohols.
- Carboxylic acids are stronger acids than phenols.
- Carboxylic acids are stronger than peroxy acids.
- Formic acid is stronger is a stronger acid than benzoic acid.
Key Reactions
Perkin Condensation
A reaction between an aromatic aldehyde with an anhydride in an alkali salt of the acid gives α,β-unsaturated carboxylic acids. This is utilized in infinitely many such instances, including in synthesizing cinnamic acids, which are precursors to a wide range of perfumes and pharmaceuticals.
Aromatic aldehydes when heated with the anhydride of an aliphatic acid (containing two $\alpha$-H atoms) in the presence of its sodium or potassium salt result in condensation to form α,β-unsaturated acid.
Mechanism

For example,

Reformatsky Reaction
The Reformatsky reaction This is the reaction of different α-halo esters and appropriate aldehydes or ketones in the presence of a zinc metal. The products for this kind of reaction include the β-hydroxy esters, which are very vital in the sense that they act as an intermediate in the synthesis of several biologically active compounds.
Ketones and aldehydes react with α-bromoesters(BrCHRCOOEt) and Zn in benzene to form $\beta$-hydroxy esters. First, the zinc organometallic BrZnCHRCOOEt is formed and then it adds to the ( $\mathrm{C}=0$ ).
Mechanism

For example,

Benzoin Condensation
Benzoin Condensation is a process whereby the reaction of two aromatic aldehydes in the presence of cyanide ions ultimately yields compounds called benzoins. This reaction becomes of immense importance in the synthesis of aromatic ketones and alcohols.
Benzil-Benzilic Acid Rearrangement
The Benzil-Benizilic Acid Rearrangement is a process whereby, upon treatment with a base, benzil is converted into benzilic acid. A certain rearrangement constitutes the most important step in the synthesis of a wide range of α-hydroxy acids that have very extensive applications in pharmaceuticals and cosmetics.
Benzoin condensation
When benzaldehyde is refluxed with aq. alcoholic KCN solution to give benzoin($\alpha$-hydroxy ketone), the process is called benzoin condensation.
Mechanism

For example,

Benzil-Benzilic acid rearrangement
$\alpha$-Diketones undergo a rearrangement when treated with base $(\mathrm{NaOH})$ to give -hydroxy acids.
Mechanism

For example,

Relevance and Applications
Carboxylic acids represent the main classes of compounds of great industrial and biological importance. They find broad application in the synthesis of aspirin and penicillin. It's also due to this acidic nature that carboxylic acids are used as preservatives in the food industry. This importance flows down into the synthesis of polymers, dyes, and perfumery, just to mention a few. This site presents basic knowledge of reactions and properties with elements of carboxylic acids for academic reasons, students learning organic chemistry, and researchers in further complex organic reactions and mechanisms. It is precisely the flexibility and reactivity of carboxylic acids in chemical processes that make these substances irreplaceable in most theories and applications of chemistry.
Recommended topic video on (Carboxylic Acids )
Some Solved Examples
Example 1
Question:
When CH3-CH2MgBr is treated with CO2 , CH3CH2COOH is formed, The C of -COOH group comes from -
1)methylene group
2)-CH3 group
3) from CO2 gas
4)None of these
Solution:
As we learned,
Grignard reagent with CO2 (dry ice) forms carboxylic acid via carboxylic acid salt as an intermediate.
$\mathrm{RMgX}+\mathrm{CO}_2 \rightarrow \mathrm{RCOOMgX} \rightarrow \mathrm{RCOOH}$
The carbon of the -COOH group comes from CO2 gas.
Hence, the answer is the option (3).
Example 2
Question:
In the following reaction :
$\mathrm{CH}_3-\mathrm{OH}+\mathrm{CH}_3 \mathrm{COCl} \rightarrow \mathrm{P}+\mathrm{H}_2 \mathrm{O} \rightarrow Q+R$
Q and R respectively are,
1)HCOOH + C2H5OH
2)CH3COOH + C2H5OH
3)HCOOH + CH3OH
4)CH3COOH + CH3OH
Solution:
As we learned,
Acidic and basic hydrolysis of Esters -
Acidic hydrolysis gives carboxylic acid directly. Basic hydrolysis gives carboxylates which acidify to give the carboxylic acid.
wherein

Therefore, Option(4) is correct
Example 3
Question:
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{COOH} \xrightarrow[\operatorname{red} \mathrm{P}]{\mathrm{Cl}_2} A \xrightarrow{\text { alc. } \mathrm{KOH}} B$.
What is B ?
1)$
\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{COCl}
$
2)$
\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}
$
3) $
\mathrm{CH}_2=\mathrm{CHCOOH}
$
4)$
\mathrm{ClCH}_2 \mathrm{CH}_2 \mathrm{COOH}
$
Solution:
The reaction will be -

The correct option is 3.
Summary
The unique chemical properties and considerable acidity of carboxylic acids make them central for many chemical reactions and applications. Their potential to form hydrogen bonds and esters, and to take part in reduction reactions, only adds to their variability. Some of the key reactions, like Perkin's Condensation, the Reformatsky Reaction, Benzoin Condensation, and the Benzil-Benzilic Acid Rearrangement, are just a few indicative of their critical nature in organic synthesis.
Also Read
27 Nov'24 05:52 PM
18 Oct'24 12:43 PM
18 Oct'24 12:34 PM
18 Oct'24 12:31 PM
18 Oct'24 12:27 PM
18 Oct'24 12:19 PM
18 Oct'24 12:14 PM
18 Oct'24 12:09 PM