Cathode and Anode - Overview, Differences, Charges, Uses, Conclusion
In the field of chemistry, the electrode is defined as the substance which conducts electricity. It is divided into two types cathode and anode. The cathode is the electrode where reduction takes place and the anode is where oxidation takes place in electrochemical cells. These electrode plays a very important role in the electrochemistry.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Charges :
- Uses:
- Positive and Negative Electrode :
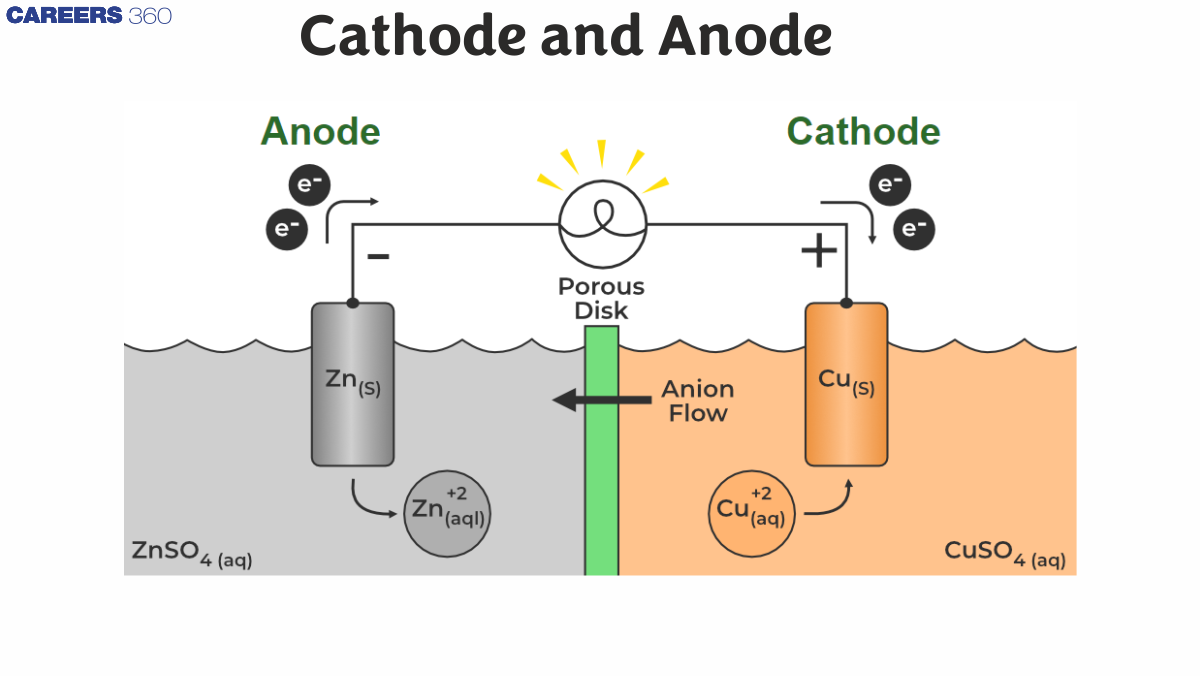
What is cathode
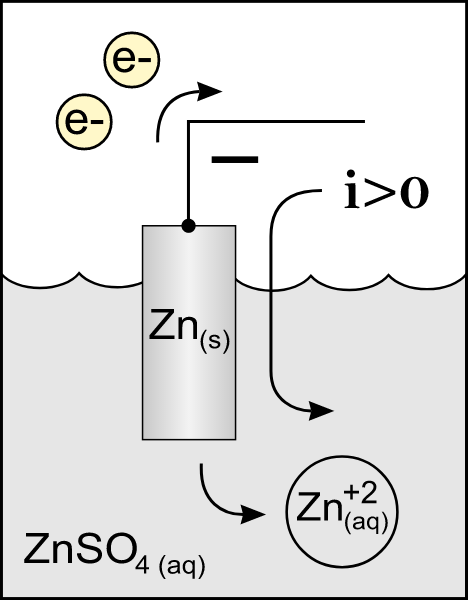
In chemistry, the cathode is defined as an electrode where reduction takes place. In an electrochemical cell. The cathode is negative because electrical energy provided in the cell causes chemical compounds to decompose. It can, however, be positive of Galvanic Cells , where chemical reaction produces electrical energy.
However, it may be positive almost like the case of an electric cell wherever the chemical process tends to the generation of power. As a result of the cathode could generate electrons, which usually square measure the electrical species doing the movement, it should be that is cathode positive generates charge current moves from the Cathode and Anode charge This could be confusing, because of the direction of current would be outlined by the manner a charge would move. simply keep in mind, that any movement of charged particles is current.
What is anode
In Electrochemistry, an anode is a place where the oxidation process happens in its most basic form. Negative ions/anions tend to react along with it give off electron at an anode because of electrical potential. The electrons then make their way up to drive circuit. In a galvanic cell, the anode is negatively charged, and the electrons tend to flow towards the outside of the circuit.
Also read :
Differences Between Anode And Cathode
Electrons can flow into an associated electrical cell or system through the cathode conductor, whereas they're going to leave from the anode charge conductor. These can modify the location below bound circumstances, i.e., Battery recharging.
The cathode can have a web charge in electrolytic cells, like a disposable battery, and an Electric Charge in galvanic cells, like a battery being recharged. Anode charge electrodes can bear the other.
Once the movement of electrons has begun within the electrical cell (discharging), the ensuing anions are going to be drawn to the anode charge finish of the cell, whereas the cations are going to be drawn to the cathode finish.
The process of electrons flowing into the cathode is thought of as reduction because it leads to a charge and a discount of the molecule’s number. Whereas with the anode charge, we are going to see an electric charge from the electrons deed, the method is thought of as oxidation.
During charging, at the cathode finish, we are going to see the creation of electrons through oxidation of anode charge material, whereas reduction of electrons at the anode charge finish. Thus, the question arises whether the anode charge is positive or negative or the cathode is positive or negative.
Related Topics |
Charges :
At the anode charge, there's an associate degree chemical reaction response. The alter species would lose the electrons, deed this conductor with associate degree negatron accumulation. Thus, the anode charge is charged. But, in distinction to the cathode, there's a discount response wherever the species of reduced ones would acquire electrons. Hence, the conductor, who suggests, the cathode, lacks electrons and is so charged absolutely.
Uses:
The Anode charge is that the negative or reducing conductor that releases electrons to the external circuit and oxidizes throughout and chemical science reaction.
The Cathode meaning is the positive or oxidizing conductor that acquires electrons from the external circuit and is reduced throughout the chemical science reaction.
Electrolyte’s area unit is typically thought of as liquids, like water or different solvents, with dissolved salts, acids, or alkalis that area unit needed for ionic conductivity. It ought to but be noted that several batteries together with the traditional.
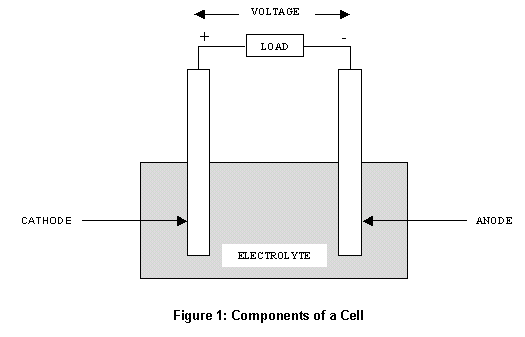
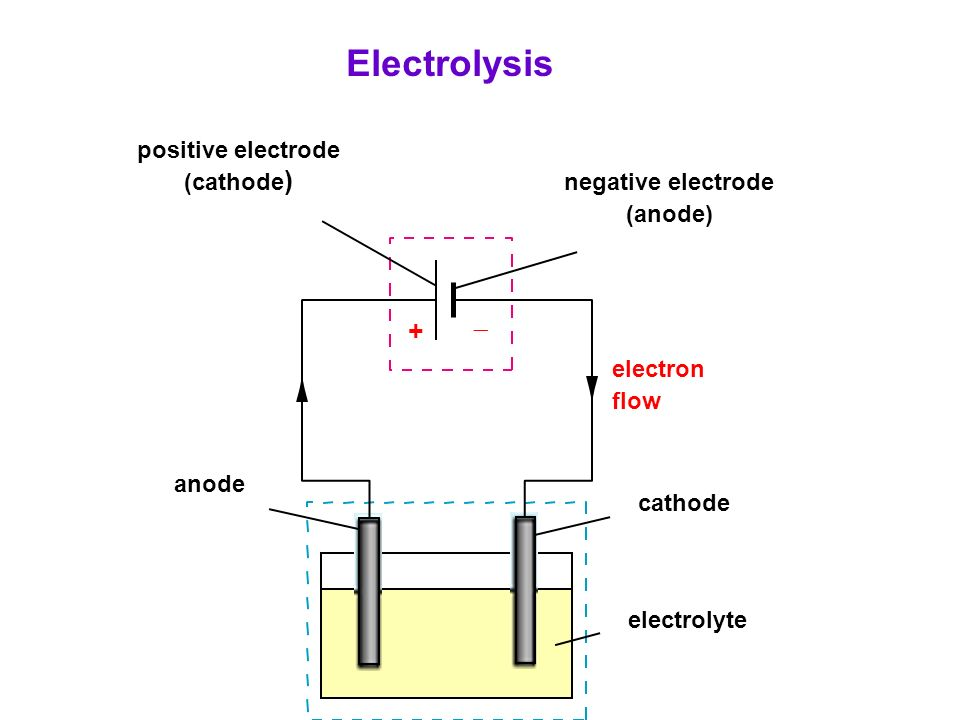
Positive and Negative Electrode :
The two electrodes of the battery or accumulator have completely different potentials. The conductor with the cathode and anode charges with upper potential is said as positive, and the conductor with the lower potential is said as negative. The electrical phenomenon, emf in V, of the battery is the distinction between the potentials of the positive and therefore the negative electrodes once the battery isn't operating.
Also read -
| NCERT notes Class 11 Chemistry | NCERT Solutions for Class 11 Chemistry |
| NCERT notes Class 12 Chemistry | NCERT Solutions for Class 12 Chemistry |
| NCERT Notes For All Subjects | NCERT Solutions for All Subjects |
Frequently Asked Questions (FAQs)
The anode charge is considered negative during a galvanic (voltaic) cell and therefore the cathode is deemed positive. This looks acceptable because of the anode charge is that the origin of electrons and wherever the electrons flow is that the Cathode and Anode charge.
During a battery (simply referred to as a galvanic cell), the anode charge is that the conductor from that the electrons leave and move into external circuit. Of course, electrons leave from negative terminal. Therefore, the solution is that the negative (-) conductor may be outlined because the anode charge, whereas the positive (+) conductor may be given as cathode. If there are any arrows given within the diagram, those represent the direction of lepton flow. "Conventional current" but, flows during a totally different direction. The anode charge exists wherever the standard current flows into the battery.
Yes, the roles of electrodes can change depending on the mode of operation. For example, in a rechargeable battery, during charging, the cathode becomes the anode and vice versa.
The differentiation depends on the type of electrochemical process:
- In galvanic cells (batteries), the anode is negative (where oxidation occurs), and the cathode is positive (where reduction occurs).
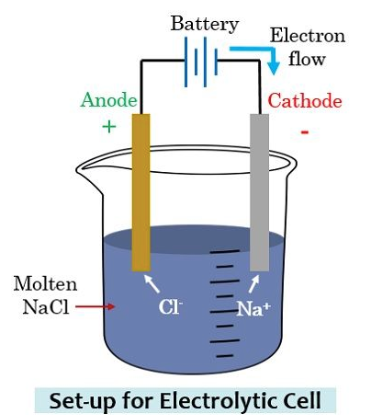
- In electrolytic cells, the anode is positive and the cathode is negative.
Electrolysis is the process of driving a non-spontaneous chemical reaction using electrical energy. In this context, the anode is where oxidation occurs (electrons are removed), and the cathode is where reduction occurs (electrons are added). The flow of electricity causes ionized substances to migrate toward the respective electrodes to undergo these reactions.
Yes, cathodes and anodes are critical components in many electrochemical processes, including electroplating, fuel cells, and corrosion protection systems (like galvanization).
Also Read
17 Dec'24 10:27 AM
12 Dec'24 01:16 PM
09 Jul'22 03:34 PM
08 Jul'22 03:43 PM
08 Jul'22 03:35 PM
08 Jul'22 03:32 PM
08 Jul'22 03:09 PM
08 Jul'22 03:03 PM
07 Jul'22 03:03 PM