Cathode Ray Experiment by Joseph John Thomson - FAQs
Introduction
Cathode ray tube experiment is the result of Sir J.J Thomson discovery of electron, full name of J.J Thomson is Joseph John Thomson. The cathode ray tube serves the function of conversion of electrical signal into visual display. The electrons emitted by a heated cathode move from atom to another atom in the form of an electric current.
In the cathode ray tube, electrons are transferred from one side of the tube towards the other end by means of electrical field. When the electrons reach towards the end, all the energy left in them id given up by the electrons because of their speed and that is transformed into other energy namely, heat. A small fraction of energy is changed into X-rays.
The discovery of electron led by sir J.J Thomson through cathode ray tube experiment is one of the most well received experiment in physics.
Also read -
Components of cathode ray tube
- Fluorescent screens
- Electron gun
- Deflection plates
Construction of cathode ray tube
The cathode ray tube is glass tube. The cathode ray is built in such a way that all the air has been drained out of the tube. The German physicist Karl Ferdinand Braun, is the inventor of cathode ray tube in such a way that the cathode rays have an evacuated glass envelope comprising of an electron gun as a source of electrons and a light which is fluorescent with means of acceleration present internally or externally to divert the electrons.
Cathode ray tube working
The beam of electrons is diverted in such a way that permit an image appearing on the projector. The image cast back electrical waves forms photographs, radar detected aircraft echoes and much more. The single ray of electrons can be transformed in a manner to show pictures in natural colours which are in movable motion.
The cathode rays consist of electrons which are negatively charged in nature.
Cathode ray tube diagram
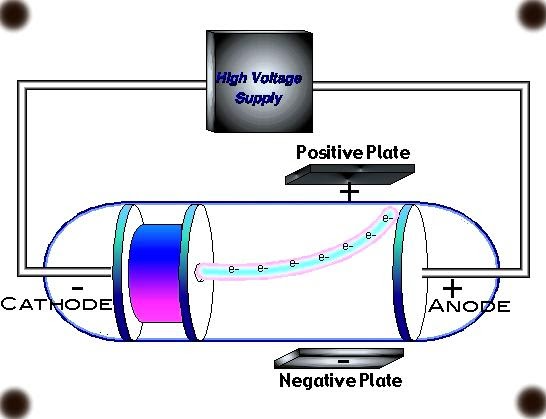
Related Topics Link, |
Set up of the cathode ray tube
The set- up of the apparatus is done is manner such that there is in co-operation of a glass tube comprising of two metal pieces towards the opposite end which behaves as electrodes. There is external voltage in connection with the two metal pieces. The air is completely drain out of the glass tube to keep the pressure of gas low.
Course of action of the experiment
- The set is made is such a way to give source of high voltage and draining the air to maintain low pressure inside of the tube.
- The metal pieces are supplied with a high voltage for air ionization to make it a good conductor of electricity.
- As the circuit is complete, the electricity starts flowing.
- A dipole is set up in experiment in addition to recognize the components of the ray given out by applying the tube a high voltage.
- On either side of the discharge ray a negative and positive pole is retained.
- The ray is repelled by the negative pole and is deflected back to the positive pole, as the dipoles are applied.
- This confirmation was further done by placing a substance which is phosphorescent in nature towards the end of the discharged ray. By noting the places carefully where the fluorescence is observed, it was pointed out that the positive side was the sight where the deflections where noted. As a result, the components of the discharge tube are charged negatively.
Also Read:
Applications of the cathode ray tube experiment
During the time unknown, the cathode ray tubes has applications in the electron beam in which there was no consideration of inactivity but have high level of frequencies and for a small fraction of time it can be made visible.
Many researchers were thrilled to get to know the secret behind the cathode rays, while many where actually in search of the applications of the cathode ray tube experiment. The introduction of Karl Ferdinand Braun’s oscilloscope ended the race of the search of all the researchers in the year 1897. The cathode ray tube had applications in production of luminescence on a screen which was chemically affected on which the cathode rays were allowed to pass through the narrow aperture by focusing on the dot look alike beam. For scanning, the dot was passed across the screen which was visually represented by the electrical pulse generator.
In twentieth century, in the first two three decades researchers kept continuing their search for applications of the cathode ray tube technology. A.A Campbell got motivated by Braun’s oscilloscope, gave counselling that cathode ray tube can be used for forecast of video picture on the screen. But on that time, Campbell-Swinton vision did not come true. In the year 1922, Philo T. Farnsworth invented a magnet which concentrated on the streamlet of electrons for fabricating a picture on the screen.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: