Celsius to Kelvin - Overview, Formula, Features, FAQs
The Celsius and Kelvin scales are two commonly used temperature measurement systems in science and everyday life. While Celsius (°C) is widely used for everyday temperature readings, Kelvin (K) is the standard unit of temperature in the International System of Units (SI). The Kelvin scale is absolute, starting at absolute zero, where all molecular motion stops, while the Celsius scale is based on the freezing and boiling points of water. Understanding the relationship between these scales is essential for converting temperatures accurately in scientific calculations.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Absolute Zero
- Temperature Conversions
- Some Solved Examples
Absolute Zero
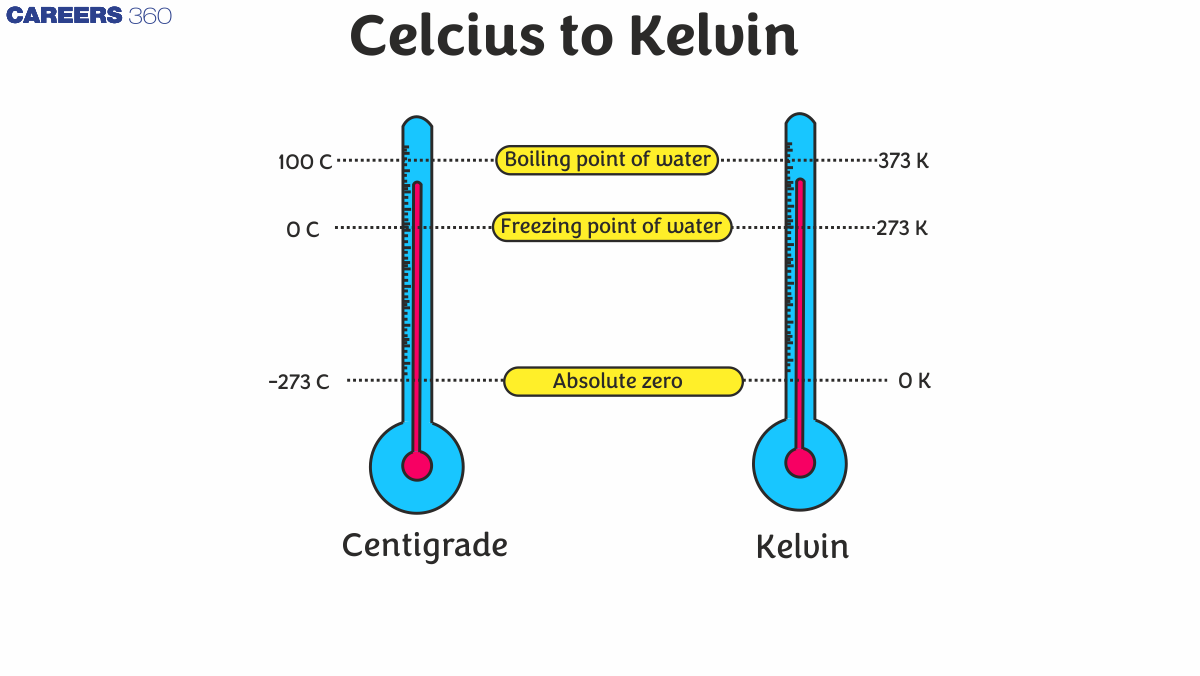
Absolute zero occurs at a temperature of 0 degrees Kelvin, or -273.15 degrees Celsius, or at -460 degrees Fahrenheit. It is a theoretical point at which molecular motion ceases.
Also read :
NCERT solutions for Class 11 Chemistry Chapter 6 Thermodynamics | |
NCERT Exemplar Class 11 Chemistry Solutions Chapter 6 Thermodynamics |
Temperature Conversions
The following is a list of conversion formulas
Celsius into Kelvin, K = 0C + 273.15
Celsius into Fahrenheit, F = (9/5) 0C + 32
Fahrenheit to Celsius, 0C = (5/9) (F-32)
Fahrenheit to Kelvin, K = (5/9) (F + 459.67)
Fahrenheit to Rankin, R = F + 459.67
Positions in Kelvin, K = (5/9) R
Freezing point of water=273.15K=00C=320F
Boiling point of water=373.15K=1000C=2120F
Related Topics |
Some Solved Examples
Example 1: Convert 25°C to Kelvin.
Solution:
As we know,
K = °C+273.15K
K= 25+273.15 = 298.15 K
Hence, the temperature in Kelvin is 298.15 K.
Example 2: What is the freezing point of water in Kelvin?
Solution:
As we know, freezing point of water is 0°C.
Using formula, K = °C+273.15K
K = 0+273.15 = 273.15 K
Hence, the freezing point of water is 273.15 K.
Example 3: Convert the boiling point of water, 100°C, to Kelvin.
Solution:
As we know,
K = °C+273.15 K
K = 100+273.15 = 373.15 K
Hence, the boiling point of water is 373.15 K.
Example 4: The surface temperature on Pluto can drop to -230°C. Convert this to Kelvin.
Solution:
As we know,
K = °C + 273.15 K
K= −230 + 273.15 = 43.15 K
Hence, the temperature in Kelvin is 43.15 K.
Frequently Asked Questions (FAQs)
Conversion of Celsius into Kelvin:
Kelvin = Celsius + 273.15.
No, it is not possible to have a negative temperature on the Kelvin scale, also known as the absolute temperature scale. This is because absolute zero, which is 0 kelvin, is the lowest possible temperature.
Absolute zero is the lowest theoretical temperature, 0 K, or -273.15°C, where all molecular motion stops.
The value 273.15 is the difference between the Celsius scale's zero point (water's freezing point) and the Kelvin scale's zero point (absolute zero).
The Kelvin scale is preferred because it starts at absolute zero, providing a true representation of thermal energy. It is essential for thermodynamic equations where absolute temperature values are required
Also Read
19 Feb'25 12:52 PM
19 Feb'25 10:40 AM
19 Feb'25 10:36 AM
19 Feb'25 10:35 AM
19 Feb'25 10:16 AM
18 Feb'25 11:45 PM
18 Feb'25 11:43 PM
18 Feb'25 11:40 PM
18 Feb'25 11:38 PM
18 Feb'25 11:36 PM

