Chemical Properties of Metals - Non-Metals, Properties, FAQs
In this article we will discuss the properties of metals, chemical properties of metals, physical properties of metals, chemical properties of metals and non-metals. The arrangement of elements in a tabular form is known by the name periodic table and there are 115 elements present in the periodic table. There are many common features as well as different features in the properties of these elements. On this basis we can divide the elements into metals and non-metals. Metals are those elements which are present on the left hand side of the periodic table and generally are of five kinds of metals: Alkaline earth metals, Alkali metals, Transition metals, Actinides and Lanthanides.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Metals:
- Non-Metals:
- Chemical Properties of Metals
- Chemical Properties of Non-Metals:
Metals:
Metals are said to be those electropositive elements which are able to donate electrons and form positive charged ions known as protons and it will become stable in nature. The general reaction shown by any metal can be represented as:
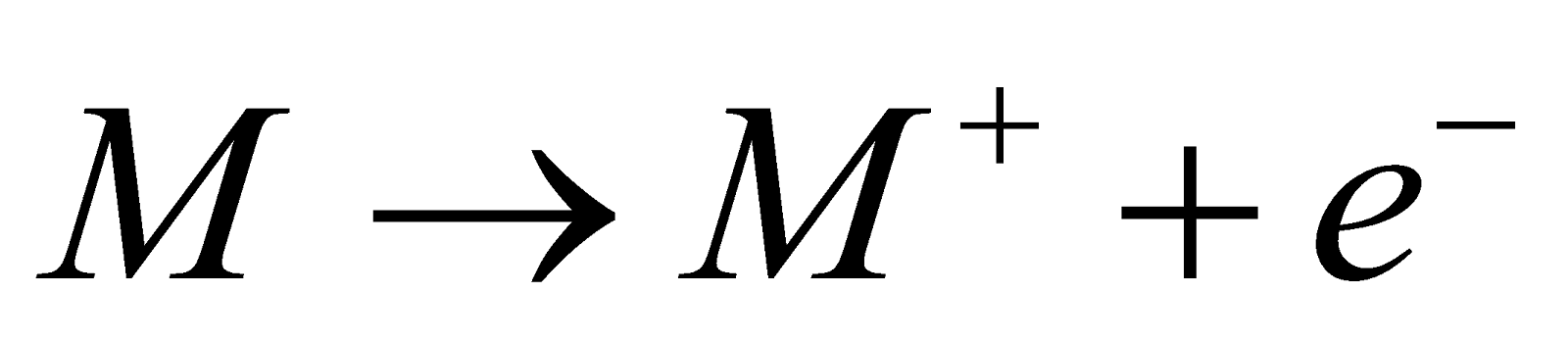 , where M stands for any metal element.
, where M stands for any metal element.
The elements which show the properties represented by the metal are come in the category of metals. Physical properties of metals are described in this manner:
1. Metals are lustrous in nature.
2. They show malleability and ductility.
3. Sonorous in nature.
4. Good conductor of heat and electricity.
5. Generally solid at room temperature.
6. High melting and boiling point.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Non-Metals:
Non-metals are said to be those elements which have tendency of accepting or gaining electrons to form negative ions. Non-metals shows 4-7 valence electrons i.e. electrons present in their outermost shell. It have high ionization energy and high electronegativity. Generally non-metals are said to be those substances which are not able to follow the properties of metals. The number of non-metals present in the periodic table are comparatively less than metals and non-metals are generally placed on the left side of the periodic table. These follows following properties:
1. Non-metals are said to be brittle and soft in nature.
2. These are poor conductor or we can termed non-metals as insulators.
3. Non-metals have no tendency to show those properties which are represented by metals like malleability, ductility etc.
And 22 non-metals are present in the periodic table.
Other than physical properties there are also some other properties called chemical properties of metals and non-metals/chemical properties of metals and nonmetals can be distinguished.
Chemical Properties of Metals
1. Reaction of metal with oxygen or reaction of metals with oxygen or Reaction of metal with air: Metals generally reacts with oxygen and form oxides known by the name metal oxides. These are of basic nature but metal oxides can also be amphoteric in nature. Amphoteric oxides are those which can behave as acid as well as basic oxides. The formation of metal oxide can be shown as:
![]()
Some metals are not fit to react with oxygen like two metals called sodium or potassium react very vigorously with oxygen and catches fire which is very dangerous and these metals are kept in kerosene oil.
2. Reaction of metal with water: When metals react with water then metal forms metal hydroxides but it is also seen that not all metals react with water. The tendency to react with water also varies from metal to metal. Metals known as sodium or potassium which reacts vigorously with oxygen are also very much reactive with water. When these metals reacts with water then it forms basic compounds or we can say alkalis. Reactions can be shown as:
a. Reaction of Sodium with water: Sodium combines with water and form sodium hydroxide and hydrogen gas which can be shown as:
![]()
b. Reaction of potassium with water: Potassium forms potassium hydroxide and hydrogen gas which can be represented as:
![]()
c. Reaction of calcium with water: Calcium when reacts with water form calcium hydroxide along with hydrogen gas which can be shown as:
![]()
Magnesium and zinc are the metals which are not able to react with cold water but can form oxides with hot water. It is the main point to remember that magnesium ad zinc form oxides with water not hydroxides. Reactions can be discussed as below
d. Reaction of magnesium with water: Magnesium forms oxides with water as compare to hydroxides and liberate hydrogen gas which can be shown as:
![]()
e. Reaction of zinc with water: Zinc also form oxide with water like magnesium and liberate hydrogen gas too. Reaction can be represented as:
![]()
Iron is very much less reactive as compare to sodium, potassium, calcium, magnesium and zinc so these are not able to react with cold as well as hot water. Iron reacts with steam and form magnetic oxides along with hydrogen gas which is represented as:
![]()
3. Reaction of metal with dilute acids: Highly reactive metals like sodium, potassium, lithium and calcium reacts very fast with dilute hydrochloric acid represented by ![]() and sulphuric acid represented by
and sulphuric acid represented by ![]() which forms salt of metal and also liberate hydrogen gas like reaction with water.
which forms salt of metal and also liberate hydrogen gas like reaction with water.
General reaction of metal with dilute hydrochloric acid can be shown as:
![]()
Reaction with dilute sulphuric acid can be shown as:
![]()
Where M represents metal ion.
Related Topics, |
On the other hand the metals which are not much reactive like magnesium, zinc, iron, lead and tin not reacts vigorously with water but show slow reactions. Like magnesium when reacts with hydrochloric acid give reaction which is as follows:
![]()
While iron reacts with sulphuric acid and gives the reaction as follows:
![]()
Metals which are present below the hydrogen atom in the series known by the reactivity series generally not able to react with dilute acids. As they do not have tendency to displace hydrogen atom which further can form a bond with any non-metal anion.
4. Reaction of metal with other salts: Metals which are very reactive in nature can easily react with those metals which are less reactive in nature. In these type of reactions more reactive metal displace the less reactive one which further form oxides, chlorides or sulphides. Reaction of metal with salts can be represented as follows:
![]()
Which metals atom will replace which atom this will be explained on the basis of electrochemical series in which metals are arranged according to their electrode potential. Electrode potential is defined as the separation between the positive and negative charges at the stage called equilibrium state results in the difference between electrical potential of metal and the solution of its ions. The potential difference of the metal at the equilibrium state depends on the nature of metal, its ions, the concentration of ions and the temperature.
Electrochemical series can be shown as follows:
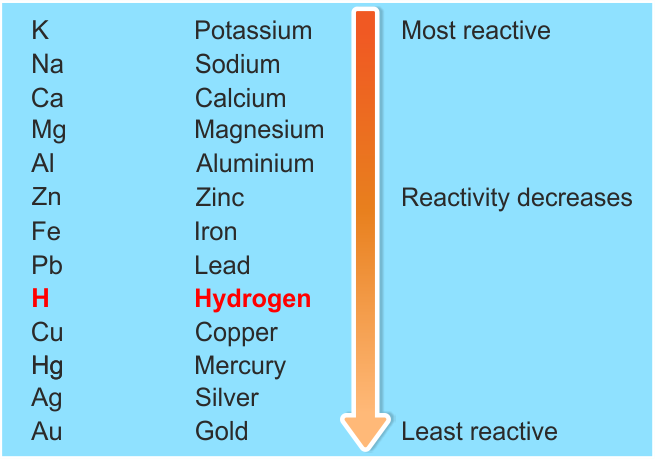
Also, students can refer,
- NCERT Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of
elements - NCERT Exemplar Class 12 Chemistry solutions Chapter 6 General Principles and Processes of isolation of
elements - NCERT notes Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of elements
Chemical Properties of Non-Metals:
1. Reactions of non-metals with water: Non-metals not generally react with water but can react with air.
2. Non-metals reaction with acids: Non-metals also not react with any acids.
3. Reaction with bases: When non-metals reacts with basis it form very complex compounds like reaction of chlorine with base let us say sodium hydroxide gives product like sodium hypochlorite, sodium chloride along with water.
4. Non-metals with oxygen/ reaction of non metals with water: Non-metals generally form oxides when react with oxygen atoms. The oxides are mainly acidic or neutral in nature.
Like when sulphur reacts with oxygen it forms sulphur dioxide which can be shown as:
![]()
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
1. Malleable
2. Ductile
3. Good conductor of heat and electricity.
Non-metals do not react with water.
Ductility is that property of metals through which metals are molded into wires. Metals like aluminium, copper, silver are drawn into wires due to these properties.
Metals generally react with oxygen and form oxides of metals along with the liberation of hydrogen gas.
Metals generally react with water and form hydroxides of metals.
Also Read
06 Feb'25 11:59 PM
06 Feb'25 11:54 PM
09 Dec'24 11:00 AM
21 Oct'24 04:24 PM
07 Oct'24 02:35 PM
07 Oct'24 02:24 PM
07 Oct'24 02:17 PM
07 Oct'24 12:55 PM
04 Oct'24 07:17 PM