Important Chemical Reactions For CBSE Class 12 - Reaction and FAQs
The reactions in which some bonds get broken towards reactant molecules and form new bonds toward product molecules which further form new substances. Reactants are those substances which are generally present on the left side and products are present on the right-hand side. There are many types of chemical reactions out of which some important chemical reactions which you all studied in class 12 are defined as follows:
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- 1. Sandmeyer Reaction:
- 2. Gattermann Reaction:
- 3. Balz-Schiemann Reaction:
- 4. Finkelstein Reaction:
- 5. Swarts Reaction:
- 6. Wurtz Reaction:
- 7. Wurtz-Fitting Reaction:
- 8. Fitting Reaction:
- 9. Friedel-Crafts Alkylation Reaction:
- 10. Frieldel-Crafts Acylation Reaction:
- 11. Riemaer-Tiemann Reaction:
- 12. Kolbe’s reaction:
- 13. Rosenmund Reaction:
- 14. Gattermann Koch Reaction:
- 15. Sabatier-Sanderson Reaction
- 16. Houben-Hoesch Reaction
- 17. Carbylamine Reaction:
1. Sandmeyer Reaction:
This is the reaction that is generally used for the preparation of aryl halides from aryl diazonium salt where aryl corresponds to the ring-like structure. Sandmeyer reaction can also be called a substitution reaction of any aromatic ring by introducing diazonium salt which is done through displacement reaction and copper is used as a catalyst in this reaction. Reaction can be shown as follows:
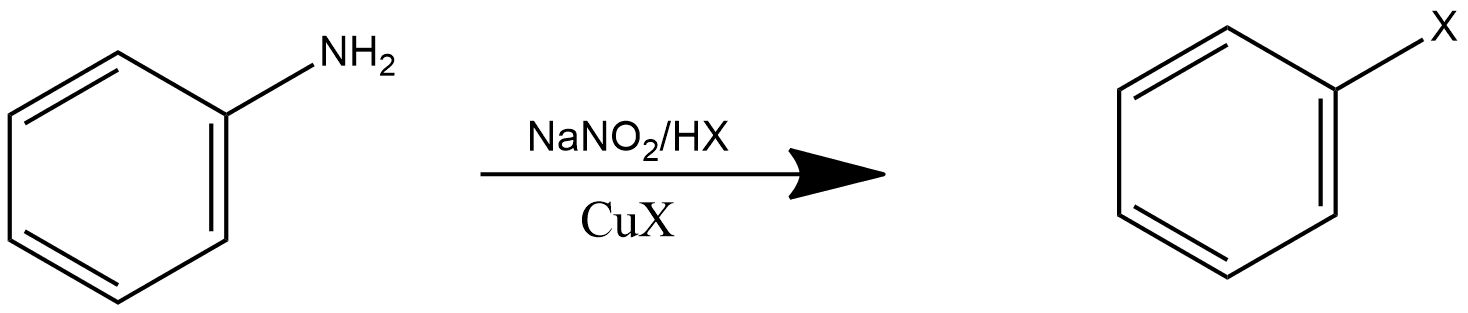
Here X represents halogen which can be chlorine, bromine, iodine, etc, or maybe a cyanide ion.
2. Gattermann Reaction:
Gattermann reaction is also known by the method of formylation of those compounds which have aromatic rings. It is very similar to Friedel-crafts reaction. This reaction is generally used for obtaining chloro or bromo benzene from bromobenzene diazonium chloride by reacting with Cu/HCl or Cu/HBr respectively and the reaction can be represented as:
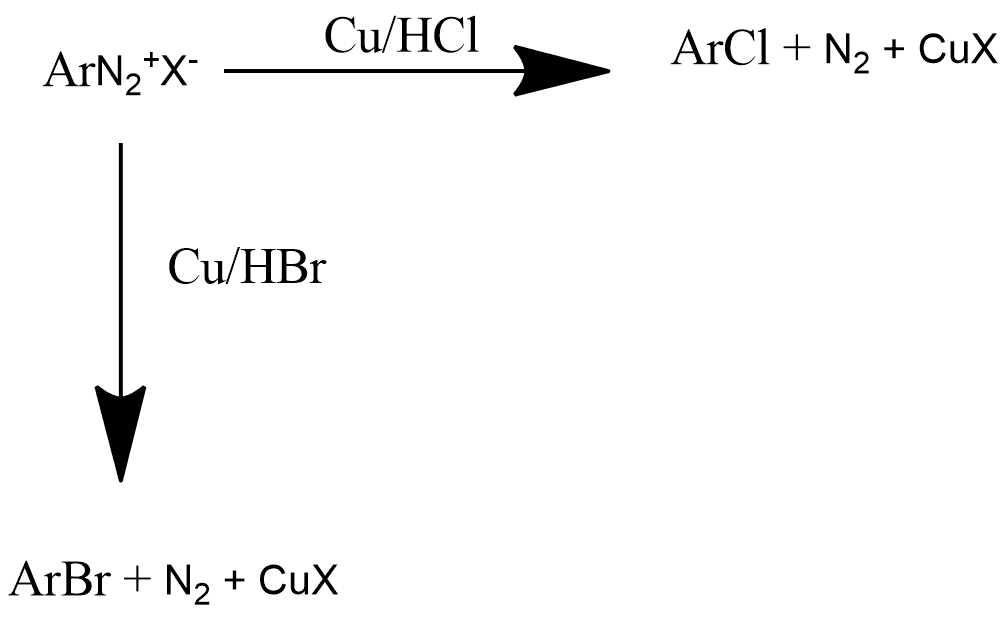
3. Balz-Schiemann Reaction:
This reaction is used for preparing aryl fluoride this can be done by a number of steps first arene-diazonium chloride is prepared with the help of fluoroboric acid then arene diazonium fluoroborate which is precipitated and decomposes further which produces aryl halide with the help of heating. Reaction can be shown as:

4. Finkelstein Reaction:
This reaction is mainly prepared for preparing alkyl iodides which can be proceeded by the reaction between alkyl chlorides with sodium iodide represented by the chemical formula NaI in the presence of dry acetone and reaction can be shown below:
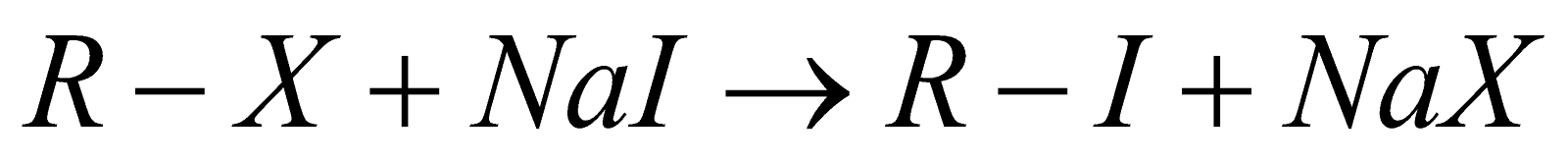
Here X represents halogen which can be chlorine, bromine, iodine, etc.
5. Swarts Reaction:
This is the reaction that gives alkyl fluoride as a product when alkyl chloride is heated in the presence of metallic fluoride compounds which can be represented as 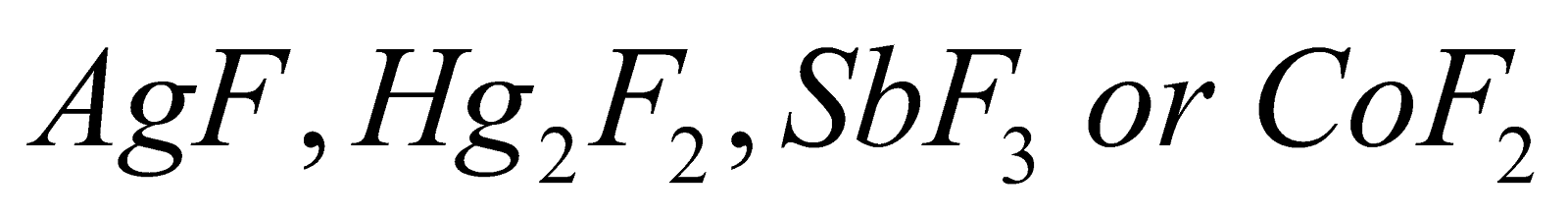 and this further gives alkyl fluorides. Reaction can be shown as:
and this further gives alkyl fluorides. Reaction can be shown as:
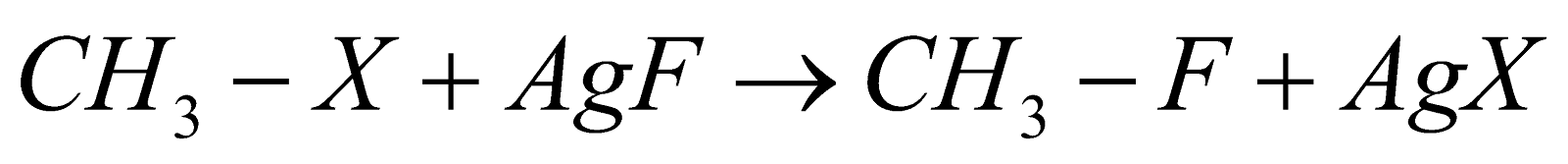
Here X represents halogen which can be chlorine, bromine, iodine, etc.
6. Wurtz Reaction:
This reaction is generally used for the production of hydrocarbons in which alkyl halides generally react with sodium and dry ether in this we get double the number of carbon atoms that we have on the reactant side and the reaction can be shown as follows:
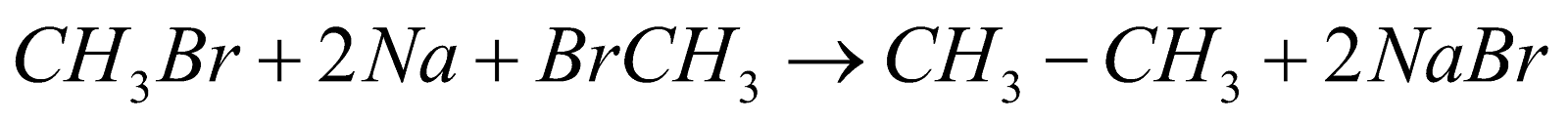
This reaction proceeds in the presence of dry acetone.
7. Wurtz-Fitting Reaction:
This reaction is one of the most basic and important reactions for getting alkyl arene. In this reaction alkyl halide or aryl halide reacts with sodium in the availability of dry ether and reaction can be represented as:
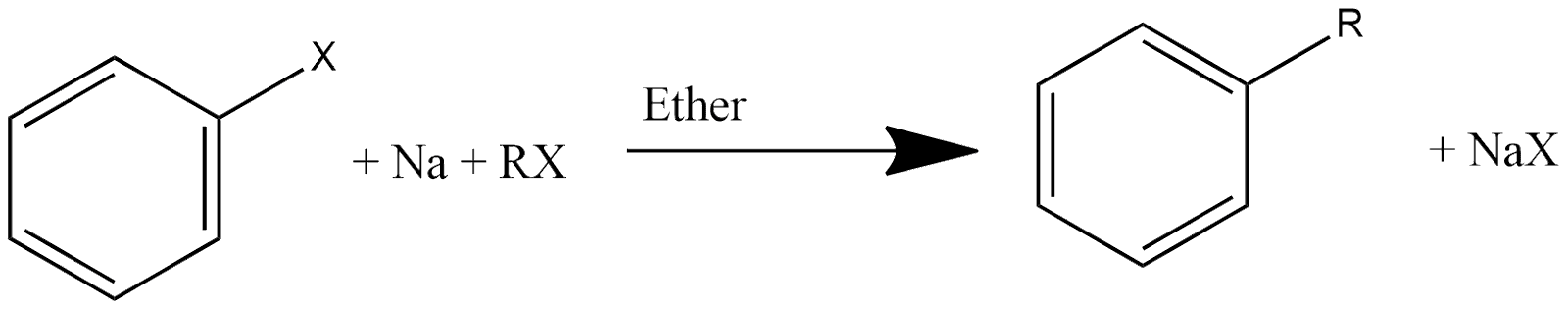
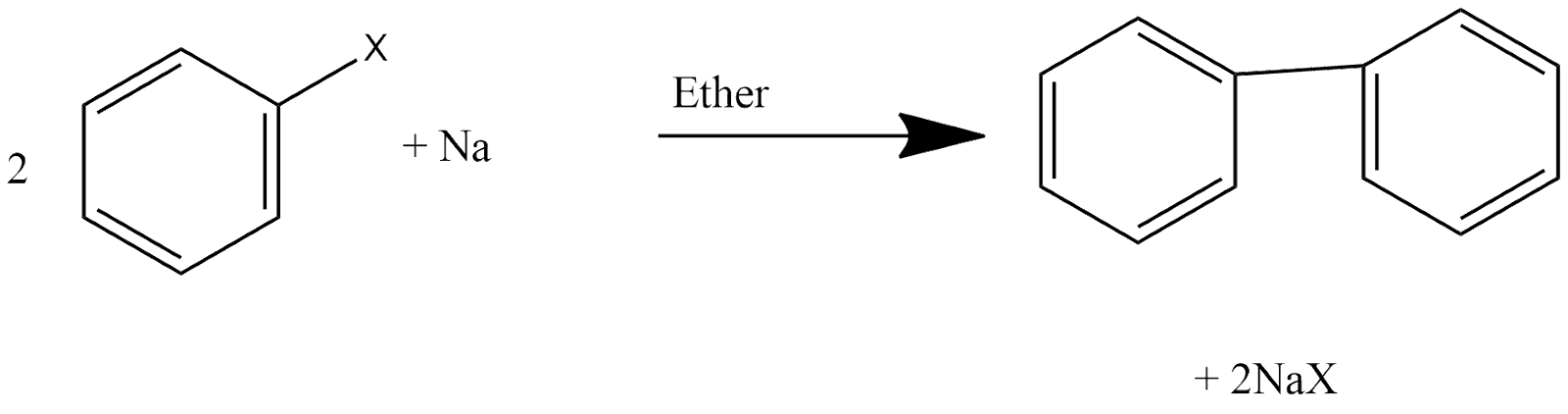
8. Fitting Reaction:
This is one of the reaction in which two aryl groups get joined with each other and we can define the reaction in such a way that aryl halides when react with sodium in dry ether which give analogous compounds in which two aryl groups are joined with each other. Reaction can be shown as:
9. Friedel-Crafts Alkylation Reaction:
In this reaction benzene is prepared with the help of an alkyl halide in the presence of anhydrous aluminum chloride represented by the chemical formula AlCl3 which further gives the alkyl benzene as a product and the reaction can be shown as:

10. Frieldel-Crafts Acylation Reaction:
Friedel craft acylation is same like friedel craft alkylation the difference is in this acyl halide is reacted with benzene but in alkyl halide alkyl halide is reacted with benzene but both the reactions takes place in presence of anhydrous aluminum chloride and the reaction can be shown as:

11. Riemaer-Tiemann Reaction:
This is a multistep reaction in which salicylaldehyde is the product of this reaction which can be shown and explained as follows:
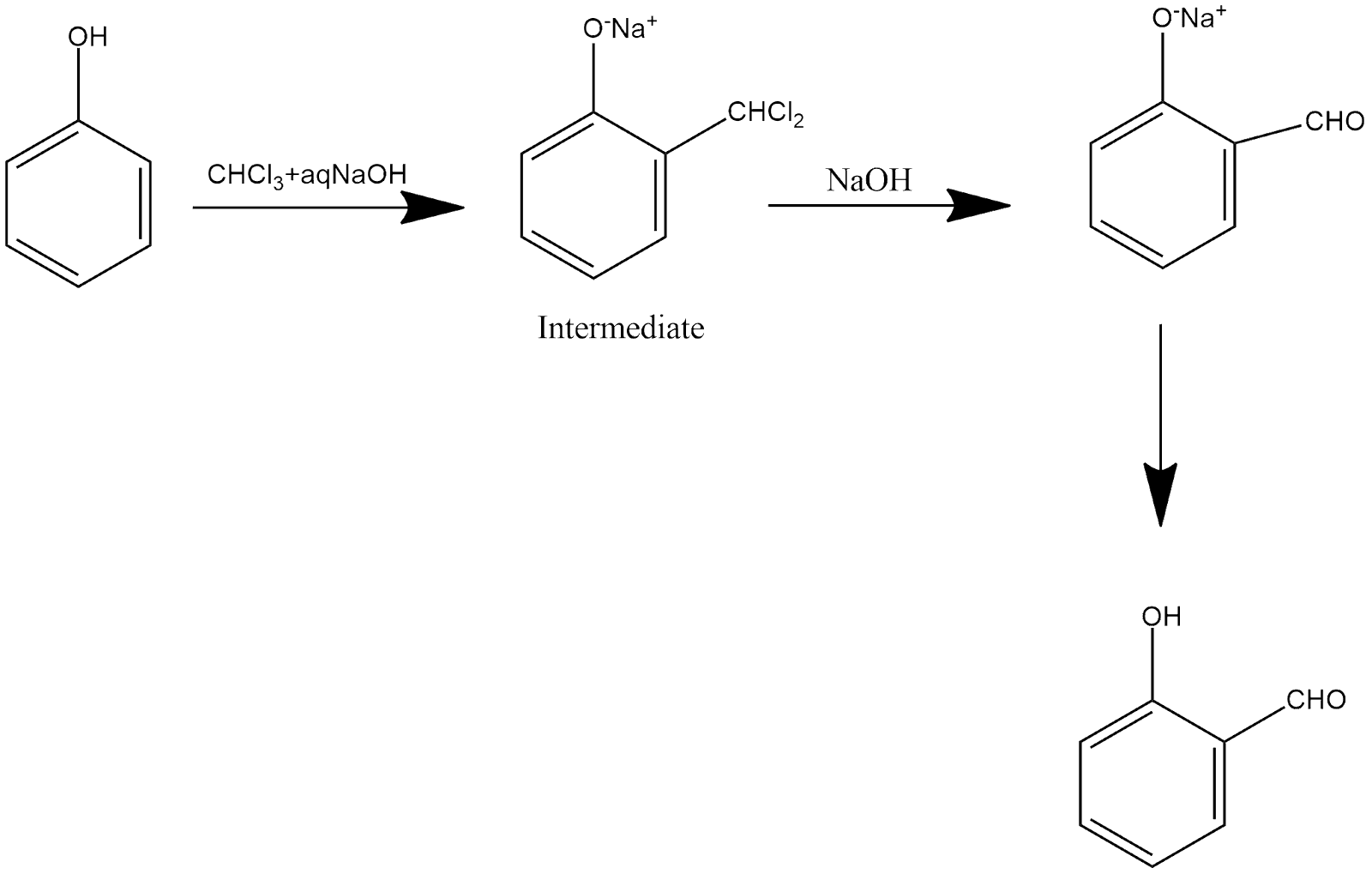
In step phenol is reacted with chloroform and sodium hydroxide and form an intermediate compound and further this reacts with sodium hydroxide in which aldehyde group is present at ortho position which on heating give salicyladehyde.
12. Kolbe’s reaction:
In presence of sodium hydroxide phenol gives sodium phenoxide which further gets reacted with carbon dioxide in acidic medium and produce hydroxybenzoic acid as a product and the reaction can be shown as:
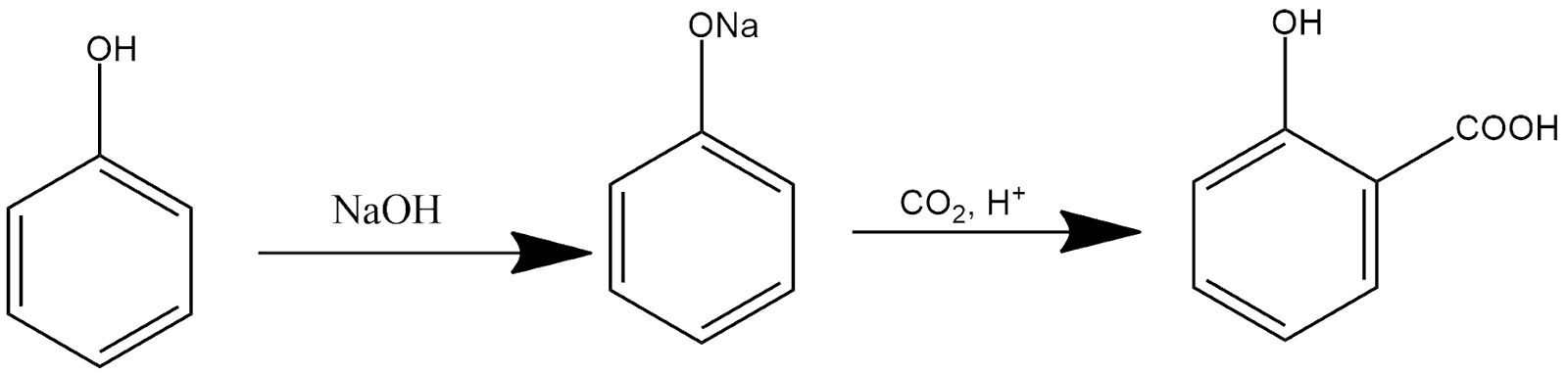
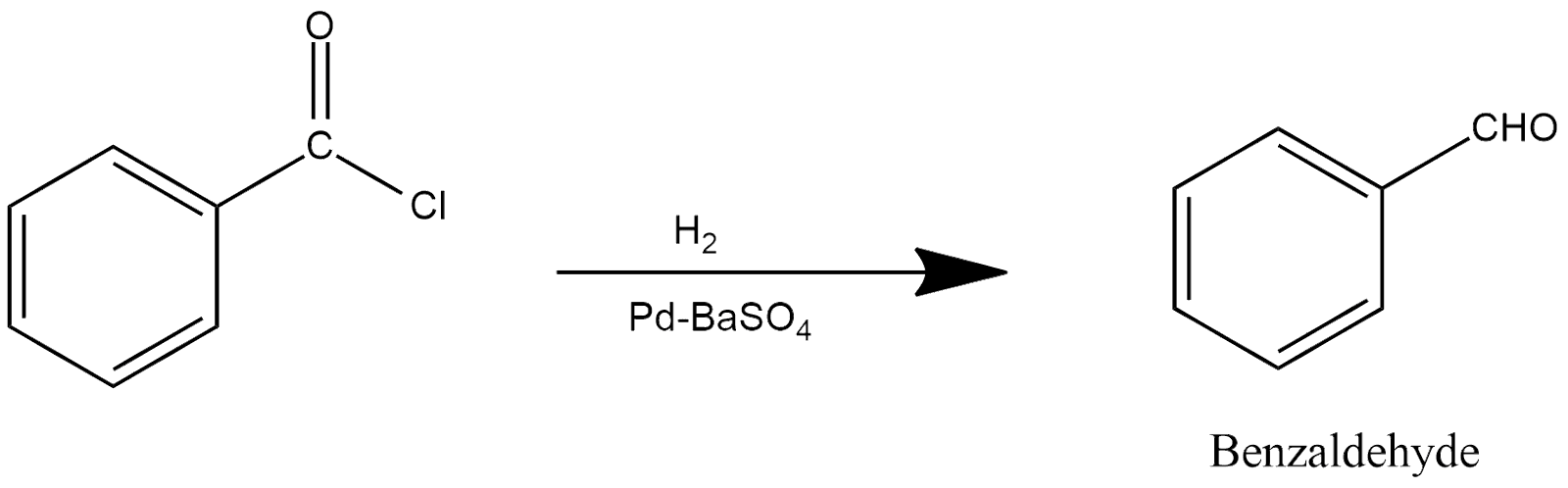
13. Rosenmund Reaction:
This reaction is generally proceed in the presence of catalyst which is known by the name of rosenmund catalyst which either be in the form of palladium or barium sulfate represented by PdSO4 or BaSO4. In this reaction benzoyl chloride undergoes through hydrogenation process and produce benzeldehyde in the presence of rosenmund catalyst and reaction can be shown as:
14. Gattermann Koch Reaction:
This reaction is also produce benzaldehyde in which benzene reacts with carbon monoxide and hydrochloric acid in the availability of aluminium chloride and gives benzaldehyde as a product which can be shown as:
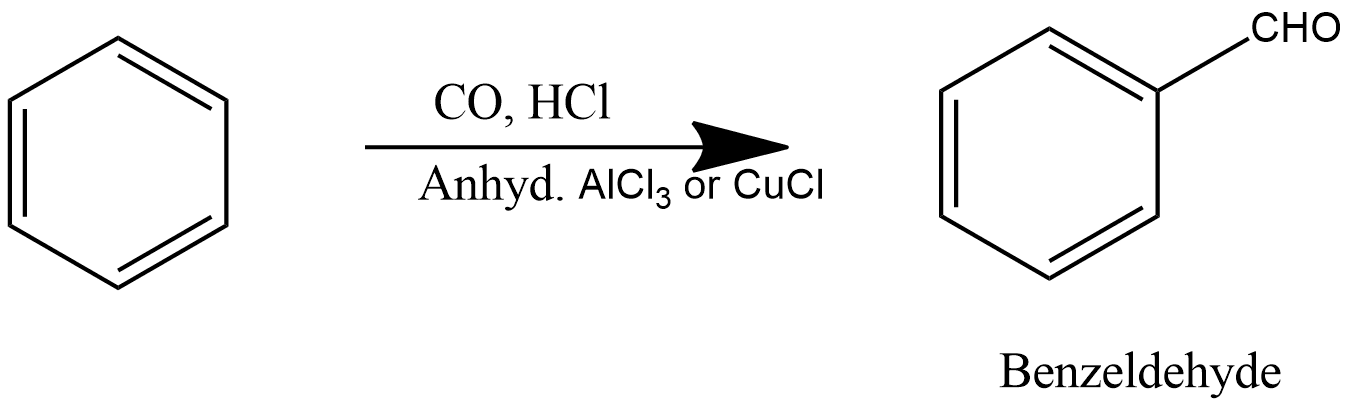
15. Sabatier-Sanderson Reaction
: In this reaction hydrogenation process occurs i.e. addition of hydrogen atoms and this reaction converts alkene into alkanes. This is one of main reaction by which ethane is prepared at large scale in this ethylene or ethane undergoes through the process of hydrogenation in the presence of catalyst Ni and gives ethane. The reaction can be represented as:
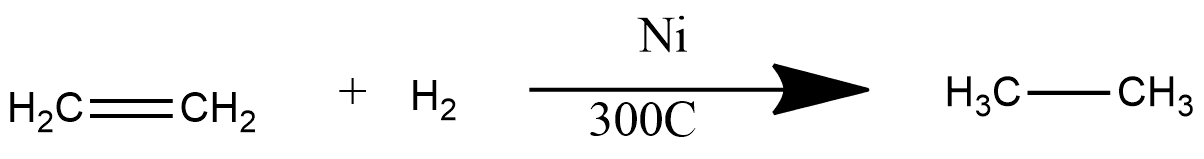
16. Houben-Hoesch Reaction
This is one of the important reaction which provide acyl chlorides as their product this is very similar with friedel craft alkylation and friedel craft acylation as we discussed above in this reaction phenol reacts with zinc chloride and cyanide group which further produces more than one acyl group in the product and the reaction can be shown as:
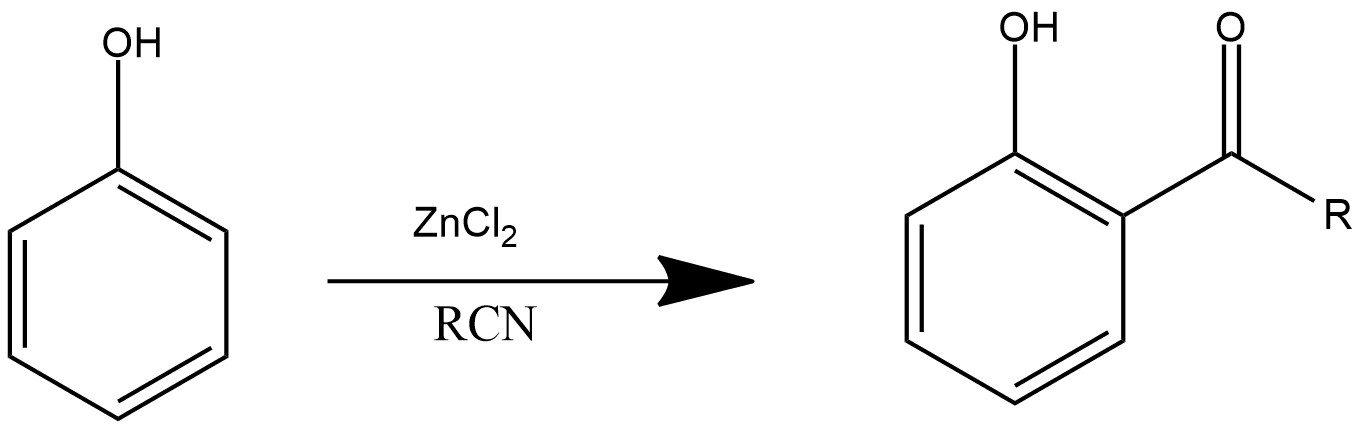
17. Carbylamine Reaction:
This is the reaction which is used to produce isocyanides or carbylamines as a product that’s why they are named as carbylamines reaction. Aliphatic and aromatic amines are heated in the presence of chloroform and ethanolic potassium hydroxide and produce isocyanides during this reaction
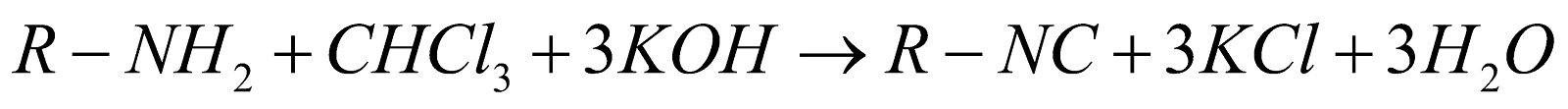
Other than these reaction many other reactions are also known to us which are aldol condensation, cross aldol condensation, gattermann Koch synthesis, Hoffmann bromaamide reaction, HVZ reaction, Fehling test, Tollen’s reagent test etc.
Also Read
| Pseudo First Order Reaction |
| First Order Reactions |
| Zero Order Reaction |
| Rate of Reaction |
| Second Order Reaction |
Also Read:
Frequently Asked Questions (FAQs)
A chemical reaction is a process that involves the rearrangement of atoms and molecules to form new substances. It involves the breaking and forming of chemical bonds.
Hell Volhard Zelinsky reaction.
Tollen’s test is basically known by the name silver mirror test in which when aldehyde gets treated with freshly prepared ammoniacal silver nitrate then it produce a mirror i.e. a bright silver mirror which represents the formation of a silver mirror.
When an aldehyde is heated with fehling solution then it produces reddish brown color precipitates.
Aldol reaction is done by those aldehydes and ketones which have one alpha hydrogen and it goes through the reaction in the presence of dilute alkali which acts as a catalyst and produce beta hydroxy aldehydes and beta hydroxyl ketones.
A chemical equation is a symbolic representation of a chemical reaction using chemical formulas and symbols. It shows the reactants, products, and their relative amounts. For example: 2H₂ + O₂ → 2H₂O