Column Chromatography - Definition, Preparation, Types, Application, FAQs
Column chromatography is a technique used for separating a single chemical compound from a mixture that has been dissolved in a fluid depending upon its polarity. It separates substances via the differential adsorption of compounds to the adsorbent, which allows them to be separated into fractions as the compounds pass through the column at various rates. This procedure can be used to purify materials that will be employed in future research on a small or large scale. This technique is an example of adsorption chromatography.
This Story also Contains
- Principles of Column Chromatography
- Preparing the Column Method
- Stationary Phase
- Mobile Phase (eluent)
- Application of Column Chromatography
- Some Solved Examples
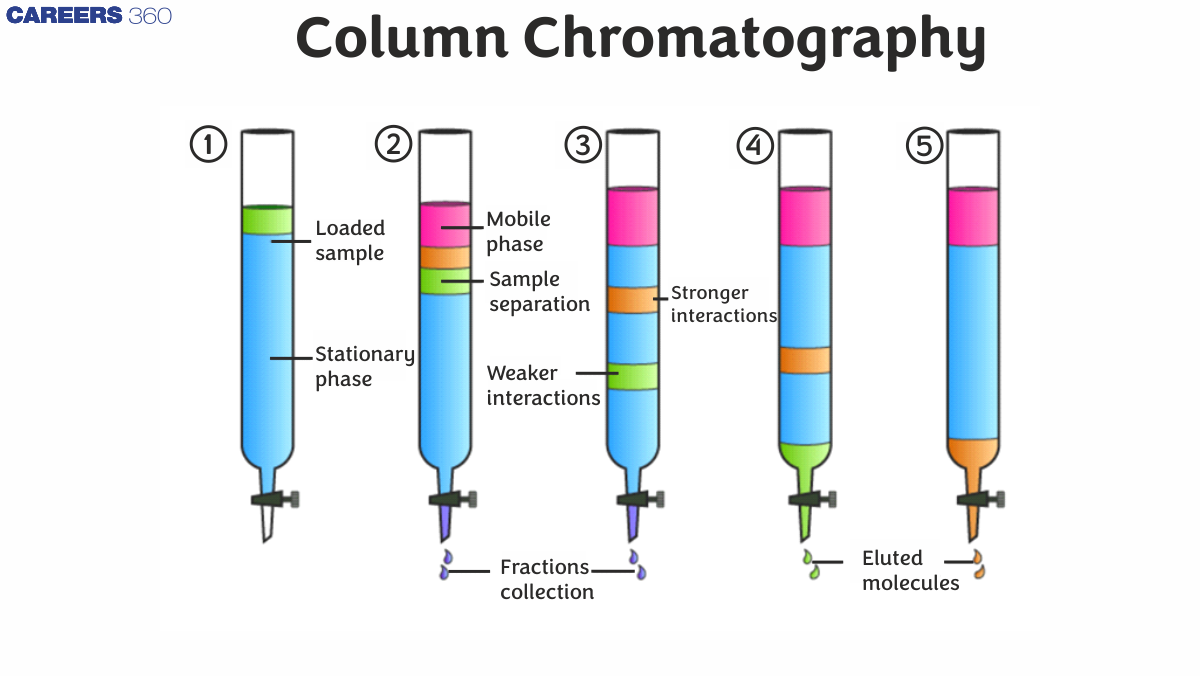
Principles of Column Chromatography
The distinct components of the mixture move at different speeds when the mobile phase and the mixture that needs to be chromatography is used to separate are injected from the top of the column. When compared to components with higher adsorption and affinity to the stationary phase, the components with lower adsorption and affinity migrate faster. The components that move quickly are removed first, followed by the components that move slowly.
The adsorption of solute molecules to the column is reversible.
The rate of movement of the components is expressed as:
Rf = the distance travelled by the solute divided by the distance travelled by the solvent.
The retardation factor is Rf.
Preparing the Column Method
A solid absorbent is packed inside a cylindrical glass or plastic tube to create a column. The size will be determined by the amount of substance that needs to be isolated. The solid phase is held in place by a filter, a cotton or glass wool stopper, or glass frit at the tube's base. At the top of the column, a solvent reservoir can be attached.
The slurry of eluent and stationary phase powder is made and gently poured into the column in the wet procedure. The top of the silica should be flat, and a coating of sand can be used to preserve the top of the silica. To progress the organic material, the eluent is slowly fed through the column.
While running at varying speeds through the column with the eluent, the distinct components are maintained differentially by the stationary phase and chromatography is used to separate them from one another. They elute one by one at the end of the column. The eluent is collected in a number of fractions throughout the chromatography process. Fraction collectors can be used to gather fractions automatically. By running many columns at once, chromatography productivity can be boosted. Multi-stream collectors are employed in this situation.
The eluent flow's composition can be monitored, and each fraction can be examined for dissolved substances using analytical chromatography, UV absorption spectra, or fluorescence, among other methods. Through the glass wall, coloured chemicals (or fluorescent compounds with the help of a UV lamp) appear as moving bands.
Also, check-
Stationary Phase
-
In column chromatography, the stationary phase or adsorbent is a solid.
-
Silica gel is the most frequent stationary phase for column chromatography, followed by alumina.
- In the past, cellulose powder was commonly utilised. Ion exchange chromatography, reversed-phase chromatography (RP), affinity chromatography, and expanded bed adsorption can all be done with a variety of stationary phases (EBA).
- The stationary phases are commonly finely powdered powders or gels that are micro porous for enhanced surface area, though EBA uses a fluidised bed. The dry weight of the analyte combination that can be added to the column has a significant relationship with the stationary phase weight. This ratio varies between 20:1 and 100:1 in silica column chromatography, depending on how close the analyte components are eluted from each other.
Mobile Phase (eluent)
- The mobile phase, also known as the eluent, is a solvent or a mixture of solvents that is used to transport molecules through the column.
-
In order to limit the time and volume of eluent required to conduct the chromatography, it is chosen so that the retention factor value of the compound of interest is roughly about 0.2-0.3.
- The eluent was also chosen to allow for the successful separation of the various components.
- In small-scale pre-tests, the eluent is optimised, often using thin-layer chromatography (TLC) with the same stationary phase.
- For each separation, there is an optimal flow rate. A faster eluent flow rate reduces the time it takes to run a column and, as a result, diffusion, resulting in improved separation. The maximum flow rate is limited, however, because the analyte must equilibrate between the stationary and mobile phases for a finite time (see Van Deemter's equation).
- A simple laboratory column is powered by gravity. The flow velocity of such a column can be increased or decreased by extending the fresh eluent-filled column above the top of the stationary phase.
Related Topics
Application of Column Chromatography
- Active compounds are isolated using column chromatography.
- It aids in the separation of compound mixtures.
- It's utilised to figure out how much a medicine costs based on its formulation.
- It's also used to get rid of contaminants.
- Metabolites are isolated from bodily fluids using this method.
Also read -
Some Solved Examples
Question 1: The efficiency of column chromatography is often represented by:
1) Retention time
2) (correct) Peak width
3) Elution volume
4) Column diameter
Solution:
The peak width of the separated components in the chromatogram often represents the efficiency of column chromatography.
Hence, the answer is option (2).
Question 2: Which of the following is an advantage of preparative column chromatography over analytical column chromatography?
1) Faster separation
2) Higher resolution
3) (correct) Larger sample loading capacity
4) Simpler instrumentation
Solution:
Preparative column chromatography has a larger sample loading capacity, allowing for the purification of larger amounts of the desired compound.
Hence, the answer is option (3).
Question 3: Which of the following is NOT a type of column chromatography?
1) (correct) Gas chromatography
2) High-performance liquid chromatography (HPLC)
3) Thin-layer chromatography (TLC)
4) Flash chromatography
Solution:
Gas chromatography is a separate technique that involves a gaseous mobile phase and is not a type of column chromatography.
Hence, the answer is option (1).
Question 4: Which of the following factors affects the retention time of components in column chromatography?
1) (correct) Mobile phase composition
2) Column diameter
3) Ambient temperature
4) Sample volume
Solution:
The retention time of components in column chromatography is affected by the composition of the mobile phase, particularly its polarity and solvent strength.
Hence, the answer is option (1).
Question 5: Which of the following factors does NOT influence the separation in column chromatography?
1) Flow rate of the mobile phase
2) Particle size of the stationary phase
3) (correct) Temperature of the column
4) Sample volume loaded on the column
Solution:
Temperature typically has minimal influence on the separation in column chromatography.
Hence, the answer is option (3).
Practice More Questions With the Link Given Below
Frequently Asked Questions (FAQs)
The basic premise of column chromatography is to use a stationary phase to adsorb solutes from a solution and then separate the mixture into discrete components.
It's a technique for purifying chemicals based on their hydrophobicity or polarity that's utilised as a precursor. The molecular mixture is separated in this chromatography technique based on its differential partitioning between a stationary phase and a mobile phase.
The main advantage of column chromatography is the low cost and ease with which the stationary phase used in the process may be disposed of. Cross-contamination and stationary phase degradation owing to recycling are avoided with the latter. Using gravity to transport the solvent through the column or pressurised gas to push the solvent through the column are two options for column chromatography.
The main components of a column chromatography setup include:
- Column: A vertical tube that holds the stationary phase.
- Stationary Phase: The material packed inside the column (e.g., silica gel).
- Mobile Phase: The solvent or mixture of solvents that flows through the column.
- Sample: The mixture that is to be separated.
- Fraction Collector: A device that collects the eluted fractions separately.
Non-polar chemicals are the answer. When compared to non-polar molecules, polar compounds will strongly commune with silica.
