Critical Temperature - Overview, Definition, Substances, FAQs
As the temperature of a gas rises, liquefaction becomes more difficult because higher and higher pressures are necessary to counteract the molecules' increasing kinetic energy. In fact, regardless of pressure, every substance has a temperature above which it can no longer be liquefied. The molecules have too much kinetic energy above the critical temperature for the intermolecular attraction interactions to keep them together in a separate liquid phase. Instead, the substance condenses into a single phase that totally fills the capacity of the container.
This Story also Contains
- Critical Temperature
- Critical Pressure
- Critical Temperatures and Pressures of Some Substances
- Some Solved Examples
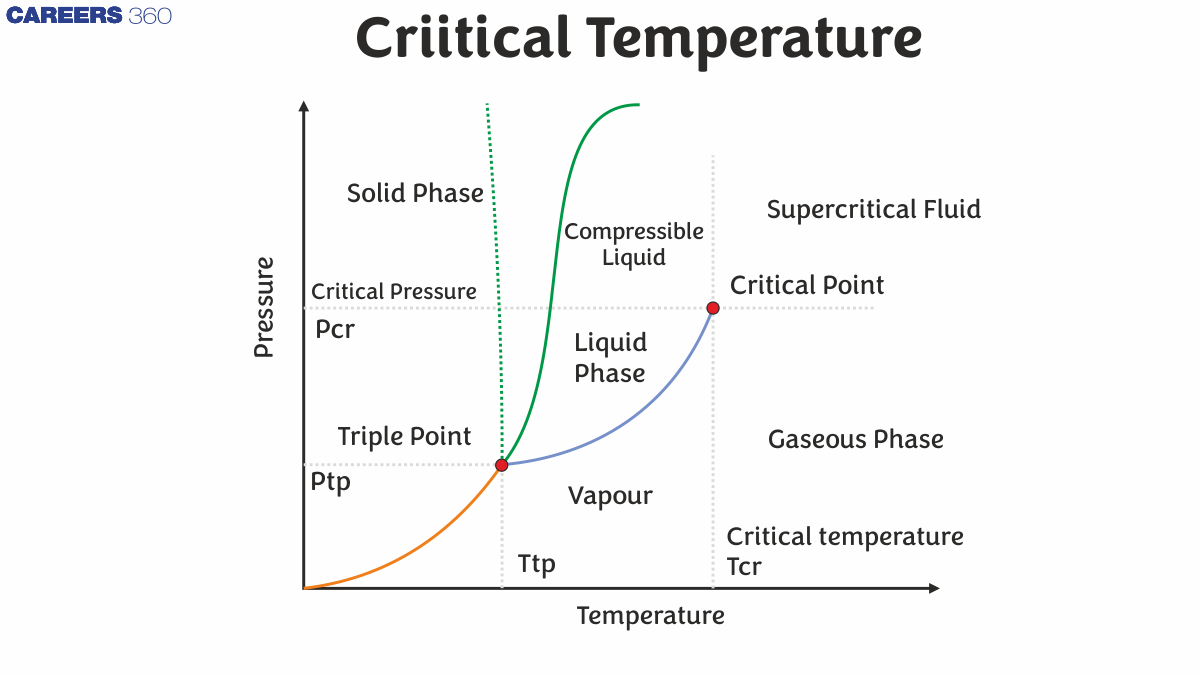
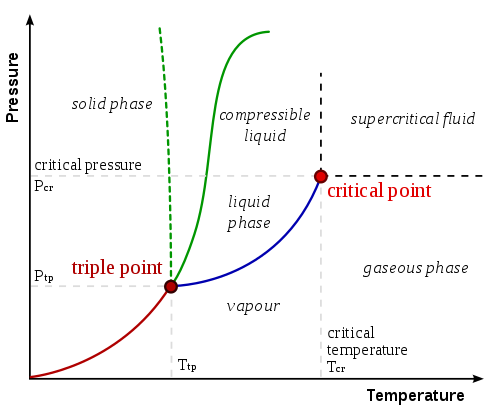
Regardless of the applied pressure, a substance cannot form a liquid above its critical temperature. The molecules have enough kinetic energy above the critical temperature to overcome the intermolecular attraction interactions. The critical pressure is the minimal pressure required to liquefy a substance at its critical temperature. A substance's critical point is the sum of its critical temperature and critical pressure. A material exists as a dense fluid called a supercritical fluid above the critical temperature and pressure, which resembles a gas in that it entirely fills its container but has the density of a liquid.
Critical Temperature
A substance's critical temperature is defined as the maximum temperature at which it may exist as a liquid. The substance in question (in its vapour/gaseous condition) can no longer be liquefied at temperatures above the critical temperature, regardless of the amount of pressure applied to it.
Critical Pressure
The pressure that corresponds to a substance's critical point is known as the critical pressure (or critical state). A material's critical point is the temperature and pressure scale point at which a liquid substance and its vapour may coexist. A substance cannot be liquefied with any amount of pressure at temperatures above its critical temperature. A substance's critical temperature can thus be described as the pressure that must be applied to a substance in order for it to liquefy at its critical temperature. The sign ‘PC' is frequently used to represent a substance's critical pressure.
Phase boundaries are recognised to separate the three most frequent forms of matter, namely solid, liquid, and gas. These phase boundaries are determined by the two fundamental factors that influence a substance's physical state — temperature and pressure. As a result, adjusting the temperature-pressure combinations can aid in determining a phase boundary. A substance's triple point, for example, is the point at which it can exist in all three states (solid, liquid, and gaseous). The temperature and pressure values at the triple points of various substances vary. The triple point of water, for example, corresponds to 0.01 degrees Celsius (or 273.16 Kelvin) and 4.58 mm of Hg. Critical pressure is an important aspect in understanding other thermodynamic phenomena.
Similarly, the phase boundary that separates a substance's liquid and vapour states can be calculated by finding a comparable temperature-pressure combination. This temperature-pressure combination is frequently referred to as the substance's critical point. The critical pressure of a substance is the pressure that corresponds to its critical point.
|
Related Topics |
Critical Temperatures and Pressures of Some Substances
The critical temperatures and pressures of several compounds are listed in a tabular column below. It should be noted that the temperature value corresponding to a substance's critical point is denoted by Tc, while the accompanying pressure is marked by Pc.
|
Substances |
Critical Temperature (Tc) |
Critical Pressure (Pc) |
|
NH3 |
132.4${ }^{\circ} \mathrm{C}$ |
113.5 |
|
CO2 |
31.0${ }^{\circ} \mathrm{C}$ |
73.8 |
| $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}$ |
240.9${ }^{\circ} \mathrm{C}$ |
61.4 |
|
He |
-267.96 ${ }^{\circ} \mathrm{C}$ |
2.27 |
|
Hg |
1477 ${ }^{\circ} \mathrm{C}$ |
1587 |
|
CH4 |
-82.6 ${ }^{\circ} \mathrm{C}$ |
46.0 |
|
N2 |
33.9 ${ }^{\circ} \mathrm{C}$ |
33.9 |
|
H20 |
217.7 ${ }^{\circ} \mathrm{C}$ |
217.7 |
|
Cl |
143.9 ${ }^{\circ} \mathrm{C}$ |
76.0 |
|
Li |
2947 ${ }^{\circ} \mathrm{C}$ |
652 |
|
Au |
6977 ${ }^{\circ} \mathrm{C}$ |
5000 |
The table above shows that metals have relatively high Tc and Pc values in general. Helium, on the other hand, has one of the lowest critical temperatures (valued at 5.19K or -267.96 ˚C).
Also check-
Critical Point
Phase barriers vanish at the critical point, which is defined by a critical temperature Tc and a critical pressure pc. Other examples include mixes' liquid–liquid critical points.
Also check-
Some Solved Examples
Question 1: The critical temperature of a gas is defined as:
A. The temperature above which gas cannot be liquefied even by applying pressure
B. The temperature at which gas liquefies at 1 atm
C. The temperature at which liquid boils
D. The temperature at which vapour pressure equals atmospheric pressure
Solution:
Critical temperature $\left(T_c\right)$ is the highest temperature at which a gas can be liquefied by pressure alone. Above $T_c$, liquefaction is impossible.
Hence, the correct answer is option (A)
Question 2: Which of the following gases has the highest critical temperature?
A. He
B. $\mathrm{H}_2$
C. $\mathrm{N}_2$
D. $\mathrm{CO}_2$
Solution:
Higher intermolecular forces → higher critical temperature.
$T_c\left(\mathrm{CO}_2\right)>T_c\left(\mathrm{~N}_2\right)>T_c\left(\mathrm{H}_2\right)>T_c(\mathrm{He})$
Hence, the correct answer is option (C)
Question 3: At the critical point, which of the following becomes identical?
A. Density of solid and liquid
B. Density of liquid and vapour
C. Density of gas and solid
D. Vapour pressure and atmospheric pressure
Solution:
At the critical point, liquid and vapour phases merge, so their densities become equal.
Hence, the correct answer is option (B)
Question 4: The critical pressure of a gas is:
A. Pressure required to liquefy gas at any temperature
B. Pressure at boiling point
C. Minimum pressure required to liquefy gas at critical temperature
D. Pressure at triple point
Solution:
Critical pressure $\left(P_c\right)$ is the minimum pressure needed to liquefy a gas at its critical temperature.
Hence, the correct answer is option (C)
Question 5: Above the critical temperature, a substance exists as:
A. Liquid
B. Gas
C. Vapour
D. Supercritical fluid
Solution:
Above both critical temperature and pressure, the substance exists as a supercritical fluid, showing properties of both liquid and gas.
Hence, the correct answer is option (D)
Frequently Asked Questions (FAQs)
The critical pressure of a substance is the amount of pressure required to liquefy that substance at its critical temperature. At its critical temperature, water, for example, requires 217.7 atmospheres of pressure to liquefy (which is 647.09 Kelvin).
A substance's triple point is the temperature and pressure at which it can exist in all three states. When the absolute temperature associated with a substance equals its critical temperature and the pressure applied to it equals its critical pressure, it is said to be at its critical point.
The critical temperature of a substance is the greatest temperature at which it may exist as a liquid without becoming a gaseous substance. The critical point between the liquid and vapour phases, for example, describes the conditions under which a liquid substance can coexist with its vapour. A given gas cannot be liquefied simply by applying pressure to it at a high enough temperature point (above the critical temperature).
The critical temperature of water is 647.1 K (374°C). Beyond this temperature, water cannot exist as a liquid regardless of the applied pressure.
Supercritical fluids exist at temperatures and pressures above the critical point. They combine the density of a liquid with the compressibility of a gas and are used in applications like supercritical CO₂ extraction for caffeine removal.