Crystallization - Definition, Examples, Principles, Types, Advantages & Uses, FAQs
Have you ever wondered how pure sugar crystals are obtained from sugarcane juice? Why do impure solids form regular-shaped crystals when a hot saturated solution is cooled slowly? The answer is Crystalisation. Crystallization is the process through which a substance's atoms or molecules arrange themselves in a well-defined three-dimensional lattice, reducing the system's overall energy. When a substance crystallises, its atoms or molecules form well-defined angles that bond them together.
This Story also Contains
- Crystallisation
- Principle of Crystallisation
- Crystallization Process
- Purification Using Crystallisation
- Types of Crystallisation
- Evaporative Crystallization
- Cooling Crystallization
- Recrystallisation
- Advantages of Crystallisation
- Uses of Crystallization
- Some Solved Examples
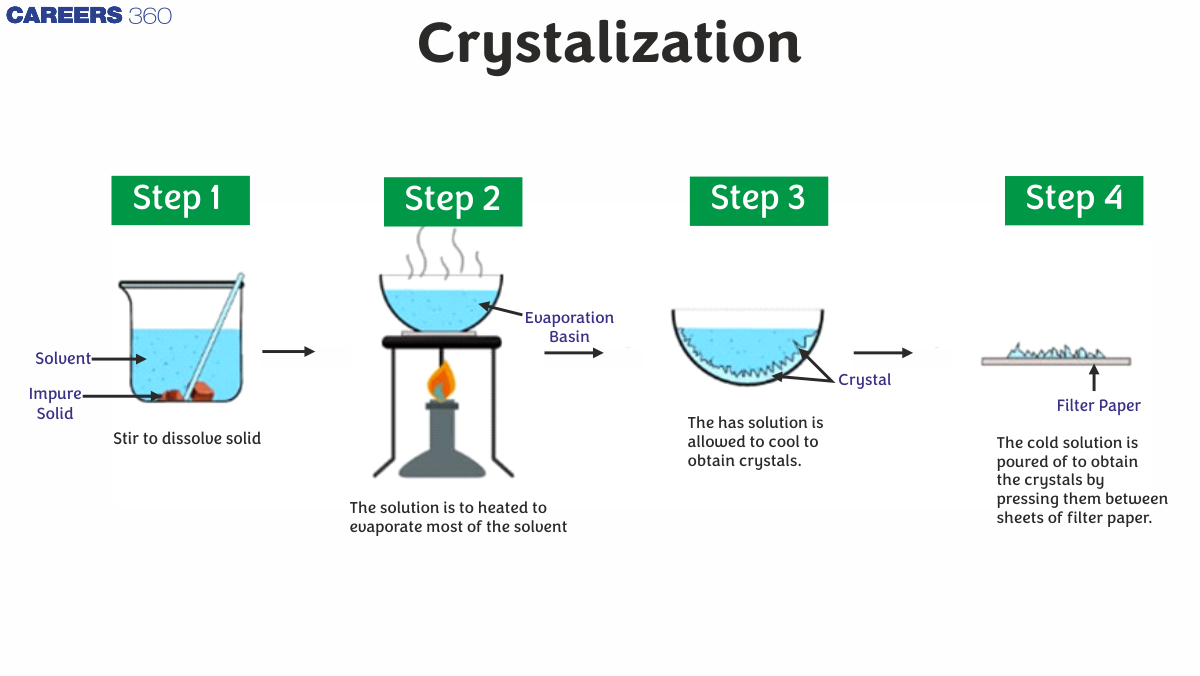
The method of crystallisation is used to purify substances. A technique for separating solids from a solution. When a solid substance is mixed with a liquid and stirred, the solid dissolves in the liquid. However, as more solid is added to the liquid, a point is reached where no more solid can dissolve. This is referred to as a saturation point, and the fluid is referred to as a saturation solution.
Crystallisation
Crystallisation is a natural occurrence that occurs when materials harden from a liquid or precipitate from a liquid or gas. A physical change, such as a change in temperature, or a chemical change, such as acidity, can be used to carry out this procedure. The size and shape of the molecules involved, as well as their chemical properties, are used to guide the crystallisation process. Crystals can be produced from a single atom, several ions, or even large molecules such as proteins. Because their internal chemistry is not symmetrical or interacts with itself to avoid crystallisation, some large molecules have a tough time crystallising.
Crystallisation Examples:
-
Water of crystallisation refers to the fixed number of water molecules contained in one formula unit of a salt. Or, to put it another way, crystallised water that is stoichiometrically linked. CuSO4.5H2O, for example, is the chemical formula for hydrated copper sulphate. With 5 molecules of water, copper sulphate crystallises.
-
Salt crystallisation is the most practical application of crystallisation today, as well as the most cost-effective method of producing salt. The method can also be used for compound purification and crystal production. Water of crystallisation can also be defined as the water molecules that make up the structure of a crystal.
-
The freezing of water to produce ice cubes and snow.
-
When honey is placed in a jar and exposed to the right circumstances, it crystallises.
-
Stalagmites and stalactites are rock formations (especially in caves).
-
Gemstone crystals are formed via a crystallisation process called deposition.
Principle of Crystallisation
The principles of solubility govern crystallisation: compounds (solutes) are more soluble in heated liquids (solvents) than in cold liquids. When a saturated hot solution cools, the solute no longer dissolves in the solvent and produces pure compound crystals.
Crystallization Process
- In an open container, the solution is heated.
-
The solvent molecules begin to evaporate, leaving the solutes behind.
-
As the solution cools, solute crystals begin to accumulate on the solution's surface.
-
Crystals are gathered and dried according to product specifications.
-
The crystallisation process of filtration separates the liquid's undissolved solids.
-
The rate of cooling determines the size of the crystals generated during this crystallisation process.
-
When a solution is rapidly chilled, a large number of small crystals develop.
-
Slow cooling rates result in the formation of large crystals.
Purification Using Crystallisation
Crystallisation is the process through which a substance's atoms/molecules arrange themselves in a well-defined three-dimensional lattice, reducing the system's overall energy. The method of crystallisation is used to purify substances. A technique for separating solids from a solution.
The crystallisation method can be used to purify mixtures that is
-
Insoluble and/or soluble contaminants are present.
-
Have a crystalline nature.
-
Filtration is ineffective because some contaminants are soluble.
Pure solids are separated from contaminants using the crystallisation procedure. For example, sea salt is separated from impurities, and alum crystals are removed from impure samples.
Types of Crystallisation
Crystallisation is broadly classified into two main branches. The following are the types of crystallisation:
-
Evaporative crystallization
-
Cooling crystallization
Evaporative Crystallization
The crystals are extracted from the evaporation of the solvent in the evaporative crystallisation process. The main liquid was suspended in a vapour as a result of this operation. The product's equilibrium concentration will remain in the main liquid. Recycling the main liquid can be used to gather the remaining product. Impurities can prevent the main liquid from being recycled. Impurity concentrations will eventually rise to the point where they will interfere with crystallisation or the purity of the product. The primary liquid stream can no longer be recycled, and the remaining liquid must be expelled.
Cooling Crystallization
Crystallisation happens mainly when the product's solubility increases dramatically as the temperature rises. Cooling crystallisation is often more energy efficient than evaporative crystallisation in these situations. The product is cooled in a heat exchanger, which might be inside the crystallizer or an external loop, in a cooling crystallisation process. The wall of the crystallizer can serve as an internal heat exchanger, or the heat exchanger can be built within the crystallizer in the form of cooling tubes or plates. When the liquid is chilled to a temperature below the equilibrium solubility, crystallisation can occur.
Melt crystallisation is a type of cooling crystallisation that occurs when a liquid is melted. The absence of solvents distinguishes cooling crystallisation from solution, indicating that most melt crystallisation procedures are carried out near the original product's melting point. An impure melt is the end product of a melt crystallisation process. Cooling this melt below the equilibrium temperature results in the creation of a solid phase that is purer than the product, whereas the impurities would prefer to remain in the impure liquid.
Also read :
Recrystallisation
Recrystallisation meaning: Recrystallisation, commonly known as fractional crystallisation, is a solvent-based method for purifying impure compounds. The purification method is based on the idea that the solubility of most materials increases as the temperature rises. This means that the amount of solute that can be dissolved in a solvent increases as the temperature rises.
Advantages of Crystallisation
The main advantages of crystallisation are as follows:
-
Through the crystallisation crystallization process, a high-purity product can be created in a single step.
-
The crystallised dry items can be packaged and stored immediately.
-
This method uses a small amount of energy and operates at a low temperature.
Uses of Crystallization
In laboratories, crystallisation is commonly used. It can be used to purify compounds and combined with modern imaging techniques to learn more about the nature of the crystalline substances. A material can be mixed into an appropriate solvent for laboratory crystallisation. Heat and acidity changes can aid in the total dissolution of the substance. The materials in the solution precipitate out at varying rates when these conditions are changed. Pure crystals of desired substances can be created under the right conditions.
Crystallography is a type of advanced imaging. High-energy beams or X-rays, as well as particles, can be blasted through the crystal structure of a pure substance using this technique. While the beams and particles do not produce a visual image, they are diffracted in certain patterns. A particular developing paper or electronic detector can identify these patterns. Mathematics and computers can be used to analyse the patterns, and a crystal structure can be created. Particles or beams are diverted by thick electron clouds in the crystal structure, resulting in diffraction patterns. These dense spots in the crystal are the atoms and bonds that formed during the crystallisation process.
Some Solved Examples
Question 1: Crystallisation is mainly used for:
A. Separation of volatile liquids
B. Purification of solids
C. Separation of immiscible liquids
D. Purification of gases
Solution:
Crystallisation is a purification technique used to obtain a pure solid from an impure solid dissolved in a solvent.
Hence, the correct answer is option (B)
Question 2: Which of the following conditions is essential for crystallisation?
A. Solubility should increase with an increase in temperature
B. Solubility should be independent of temperature
C. Solubility should decrease with an increase in temperature
D. Substance must be volatile
Solution:
For crystallisation, the solid must be more soluble in a hot solvent than in a cold solvent.
Hence, the correct answer is option (A)
Question 3: During crystallisation, impurities remain:
A. In the crystal lattice
B. On the crystal surface
C. In the mother liquor
D. As vapours
Solution:
Most impurities stay dissolved in the mother liquor, while pure crystals separate out.
Hence, the correct answer is option (C)
Question 4: Which method gives better purity of crystals?
A. Rapid cooling of hot solution
B. Evaporation to dryness
C. Slow cooling of saturated solution
D. Cooling under reduced pressure
Solution:
Slow cooling allows proper arrangement of particles into a well-ordered crystal lattice, giving purer crystals.
Hence, the correct answer is option (C)
Question 5: Which of the following is NOT a correct statement about crystallisation?
A. It is a purification method
B. It forms crystals with a definite shape
C. It separates volatile impurities
D. It involves a change of solubility with temperature
Solution:
Crystallisation removes non-volatile impurities, not volatile ones.
Hence, the correct answer is option (C)
Frequently Asked Questions (FAQs)
When a compound crystallizes from water or a water-containing solvent, water molecules become part of its crystalline structure. The water molecules are not directly bonded to the metal cation. Hence, water of crystallization is water that is chemically bonded into the crystalline structure of a compound.
Natural examples include the formation of snowflakes, mineral crystals, and salt deposits from evaporated seawater.
The application of crystallization are:
Purification of alum from impure sample
Purification of seawater
For the synthesis and isolation of co-crystals, pure active pharmaceutical ingredients (API), controlled release pulmonary drug delivery.
Separation of chiral isomers.
Crystallisation is utilised as a separation and purification method in the pharmaceutical industry.
Crystallization relies on the principle of supersaturation, where the solute concentration exceeds its solubility, causing it to form a solid.
The formation of massive crystals of pure substances from their solutions is referred to as Crystallization.
