Decomposition Reaction - Definition, Examples, Types, Uses, FAQs
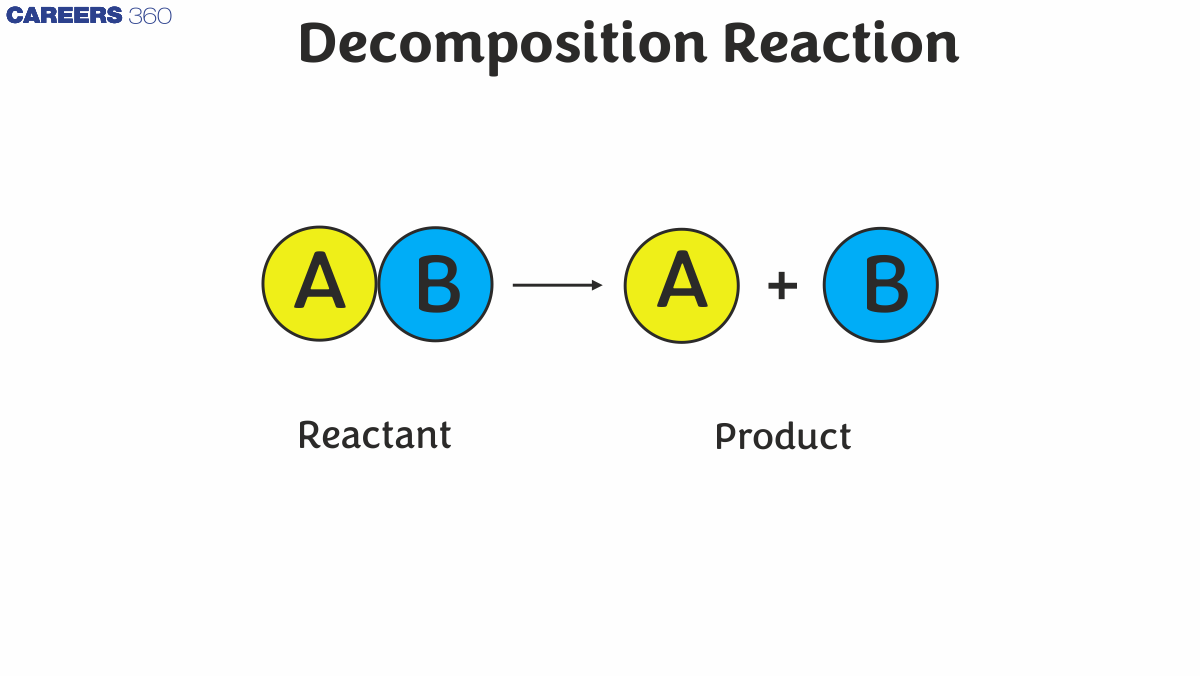
A decomposition reaction is a type of chemical reaction in which a single compound breaks down into two or more simpler substances, such as elements or smaller compounds. This process often requires an external source of energy, such as heat, light, or electricity, to break the chemical bonds. The general formula for a decomposition reaction is: AB→A+B. Here, AB is the reactant that decomposes into the simpler products A and B.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Examples of decomposition reactions
- What are the uses of decomposition reaction?
- Double decomposition reaction
- Types of Decomposition reaction
- What Happens During Response Rotation?
- How Can You Measure a Decomposition?
- Alternative Response to Decay
- Examples of decomposition reactions in Real Life
- Thermolysis
- Ease of Decomposition reaction:
Examples of decomposition reactions
- Thermal Decomposition:
- Decomposition of calcium carbonate: CaCO3→CaO+CO2
- Calcium carbonate breaks down into calcium oxide and carbon dioxide when heated.
- Electrolytic Decomposition:
- Electrolysis of water: 2H2O→2H2+O2
- Water decomposes into hydrogen and oxygen gas under an electric current.
- Photolytic Decomposition:
- Decomposition of silver chloride: 2AgCl→ 2Ag+Cl2
- Silver chloride decomposes into silver and chlorine gas in sunlight.
- Decomposition of hydrogen peroxide: 2H2O2→2H2O+O2
- Hydrogen peroxide decomposes into water and oxygen.
What are the uses of decomposition reaction?
One of the main uses for the decomposition reaction is the extraction of metals from their ores. For example, zinc can be obtained from calamine by placing it in the reaction of decomposition. In the same way, sodium can be found in sodium chloride.
Double decomposition reaction
A decomposition reaction in which two reactants alternate negative and positive ions and a new chemical is formed is known as double decomposition reaction.
Types of Decomposition reaction
Thermal decomposition reaction
The thermal decomposition reaction can be defined as a reaction to decomposition of thermal decomposition. Such reactions are often exhausting because energy is needed to break down chemical bonds and to disassociate.
Example of thermal decomposition reaction of a hot rot reaction is given below.
CaCO3 → CaO + CO2
This process is used in the production of fast lime, which is important in many industries.
Electrolyte Decomposition reaction
Electrolytic decomposition chemistry response is a type of decomposition reaction wherein activation power is supplied within the form of electrical electricity. An electrolytic decomposition reaction which is liquid electrolysis
Example of example of electrolytic decomposition reaction:
2H2O → 2H2+O2
Photochemical decomposition
A photo decomposition reaction is type of decomposition reaction by which reactant is released to builders along with absorbing energy from photons. An example of an image decay response is decay of dioxygen and oxygen radical, as represented by the chemical equation given below.
O3+hν → O2 +O.
Use of Decomposition reaction
Making cement or calcium oxide.
Metal Processes: Extraction of metals from their oxides, chlorides, etc.
Ease of acid digestion.
Welding Thermite.
Related Topics |
What Happens During Response Rotation?
During the decomposition reaction, the bonds between the atoms drop to the original object. Atoms then reorganize to form new bonds, resulting in new materials with different properties.
Features of Rot Reaction
One responder and two or more products
It requires energy
Examples of Rotting Response: An example of a decay response is the decomposition of carbonic acid (H2CO3) into carbon dioxide (CO2) and water (H2O)
How Can You Measure a Decomposition?
To quantify the equation, we measure the number of oxygen atoms on the right of the equation and compare it with the left. We notice that there are two oxygen particles on the right and three on the left. Therefore, we multiply right-O at 3 and left to O at 2.
Note that we will increase the total KClO3 by 2 and measure oxygen.
Now, we note that potassium (K) and chlorine (Cl) are not measured. To the left of the equation, there are two K and Cl atoms, and on the right, there are only one atom. Therefore, we multiply KCl built in 2 and get the final number.
Alternative Response to Decay
Catalytic decomposition
In this type of decomposition, the reaction occurs with the help of a catalyst.
For example
The decomposition of hydrogen peroxide in water and oxygen is stimulated by an enzyme called catalase
2 H2O2 (l) + catalase (enzyme) → 2H2O (l) + O2 (g)
Also read :
| NCERT notes Class 11 Chemistry Chapter 6 Thermodynamics | |
| NCERT solutions for Class 11 Chemistry Chapter 6 Thermodynamics | |
NCERT Exemplar Class 11 Chemistry Solutions Chapter 6 Thermodynamics |
Examples of decomposition reactions in Real Life
The decay response has few applications in industry and in everyday life.
Industry:
Production of calcium oxide or quicklime
Lithium oxide production
Preparation of oxygen and carbon dioxide
In metallurgy, the extraction of metals from oxides and chlorides by electrolytic decomposition.
Daily life:
When a soda bottle is opened, carbonic acid decomposes producing water and carbon dioxide, causing fizz.
During our digestive system, carbohydrates, fats, and proteins decompose into many simple substances.
Photographic films are covered with silver bromide, which, when exposed to light, splits into silver and bromine.
When hydrogen peroxide is used to cut or wound, peroxide decomposes, and forms oxygen bubbles and explodes.
Many environmental degradations is seen in foods such as fermentation and pollution.
Food rot and vegetable peels produce excellent nutrients and soil compost.
Labs:
There are several analytical methods such as mass spectrometry, gravimetric analysis, and thermogravimetric analysis
Thermolysis
Heat decomposition, or thermolysis, is a chemical decay caused by heat. The decay temperature of an object is the temperature at which the substance decomposes chemically. The reaction often ends as heat is needed to break down the chemical bonds in the decaying area. When decay is disturbing enough, a good reaction loop is created producing a hot escape along with possibly an explosion. Some of the oxides, especially of the less fragile metals, decompose meaning when heated to a sufficient temperature. An ancient example is the decay of mercuric oxide to provide oxygen and mercury metal. When water is heated above 2000°C, a small percentage of it decomposes into OH, monatomic oxygen, monatomic hydrogen, O2, and H2.
The most common decomposing element is carbon monoxide at -3870°C (-7000°F). Decomposition of nitrates, nitrites, and ammonium compounds. Ammonium dichromate in heat produces nitrogen, water, and chromium oxide. Ammonium nitrate at high temperatures produces dinitrogen oxide ("laughing gas") and water. Ammonium nitrite in heat produces nitrogen gas and water. Barium azide in heat produces barium iron and nitrogen gas. Sodium azide at a temperature of 300 ° C provides nitrogen and sodium. Sodium nitrate in heat produces sodium nitrite and oxygen gas. Organic chemicals such as high-temperature amines cause Hofmann to dissolve and produce secondary amines and alkenes.H2O2
Ease of Decomposition reaction:
When metals are at the bottom of a series of reactivity, their compounds usually decompose easily at high temperatures. This is because the form of solid bonds between atoms at the top of a series of reactivity, and strong bonds is difficult to break.
For example, copper is near the bottom of a series of reactivity, and copper sulphate begins to decompose at about 200 ° C, rising rapidly at high temperatures to about 560 ° C. In contrast, potassium is close to the top of the series of reactivity, and potassium sulphate does not decompose where it melts at about 1069 ° C, or where it boils.
Also check-
Frequently Asked Questions (FAQs)
A decomposition reaction is a chemical reaction where a single compound breaks down into two or more simpler substances.
The main types of decomposition reactions are
1. Thermal decomposition (triggered by heat),
2. Electrolytic decomposition (triggered by electricity)
3. Photolytic decomposition (triggered by light).
The decomposition of hydrogen peroxide (H2O2) into water and oxygen is a common example, used in household antiseptics.
Answer: (b)
Decomposition of silver chloride occurs by Sunlight.
Also Read
19 Feb'25 12:52 PM
19 Feb'25 10:40 AM
19 Feb'25 10:36 AM
19 Feb'25 10:35 AM
19 Feb'25 10:16 AM
18 Feb'25 11:45 PM
18 Feb'25 11:43 PM
18 Feb'25 11:40 PM
18 Feb'25 11:38 PM
18 Feb'25 11:36 PM