Derivation of Ideal Gas Equation - Definition, Formula, Limitations, FAQs
Have you ever wondered how the pressure, volume, and temperature of a gas are connected? What single relationship can explain why a balloon expands on heating or why gases compress under pressure? The answer is the ideal gas equation. The ideal gas equation is usually called the ideal gas law. It contains in one mathematical expression the basic principles relating to pressure, volume, temperature, and number of moles of gas.
This Story also Contains
- Ideal Gas Equation
- Combined Gas Law
- Density and Molar Mass of a Gaseous Substance
- Some Solved Examples

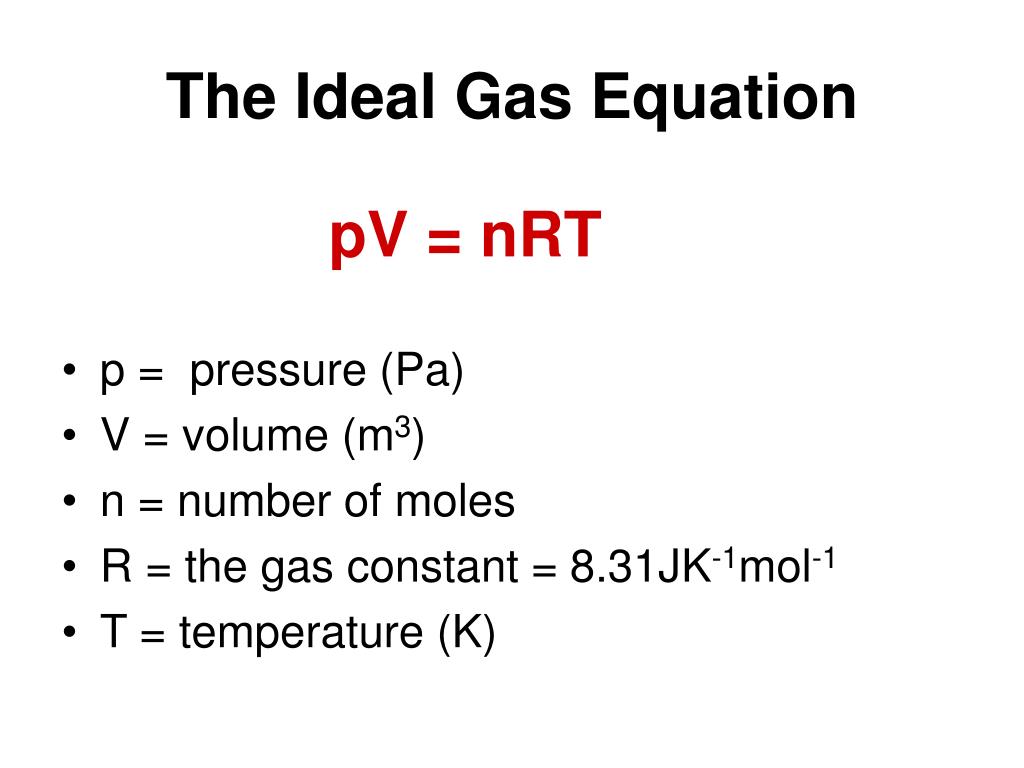
This formula is always written as
PV=nRT,
where P = pressure, V = volume, n = number of moles, R = universal gas constant, and T = Temperature in Kelvin. It is an integrated law that has Boyle’s Law, Charles’ Law, Gay Lussac’s Law, and all such laws combined into a single equation.
Ideal Gas Equation
The ideal gas equation is an equation that is followed by the ideal gases. A gas that would obey Boyle's and Charles's laws under all the conditions of temperature and pressure is called an ideal gas.
As discussed, the behavior of gases is described by certain laws such as Avogadro's Law, Boyle's Law, and Charles' Law.
According to Avogadro's Law,
$\mathrm{V} \propto \mathrm{n}$ (P and T constant )
According to Boyle's Law,
$V \propto 1 / P$ (T and n constant).
According to Charles' Law,
$V \propto T$ (P and n constant). Combining the three laws, we get:
$V \propto n T P V=R n T P$
' R ' is the proportionality constant. On rearranging the above equation, we get the following:
PV=nRT
This is the ideal gas equation, as it is obeyed by the hypothetical gases called ideal gases under all conditions
Universal Gas Constant or Ideal Gas Constant R or S: Molar gas constant or universal gas constant. Values of R=0.0821lit, atm, K−1, mol−1$=8.314$ joule $K^{-1} \mathrm{~mol}^{-1}=8.314 \times 10^7 \mathrm{ergK}^{-1} \mathrm{~mol}^{-1}=2 \mathrm{calK}^{-1} \mathrm{~mol}^{-1}$
For a single molecule, the gas constant is known as the Boltzmann constant ( k ) and unit $\left(\mathrm{m}^2 \mathrm{kgs}^{-2} \mathrm{~K}^{-1}\right)$
$k=RN0=1.38×10−3 J/deg− abs / molecule =1.38×10−16erg/ deg-abs / molecule$
Combined Gas Law
Boyle's and Charles' Law can be combined to give a relationship between the three variables P, V, and T. The initial temperature, pressure, and volume of a gas are T1, P1, and V1. With the change in either of the variables, all three variables change to T2, P2, and V2. Then we can write:
$P_1 V_1 T_1=n R$ and $P_2 V_2 T_2=n R$
Combining the above equations, we get
$P_1 V_1 T_1=P_2 V_2 T_2=n R$
The above relation is called the combined gas law.
Related Topics link
Density and Molar Mass of a Gaseous Substance
Ideal gas equation is PV=nRT
On rearranging the above equation, we get
$\mathrm{nV}=\mathrm{PRT}$
n (No. of moles )= Given mass (m) Molar mass (M)
Putting the value of ' n ' from equation (iii) in equation (ii), we get:
$\mathrm{mMV}=\mathrm{PRT}$
We know that density (d) is mass (m) per unit volume (V)
d=mV
Replacing mV in the equation. (iv) with d (density), then equation (iv) becomes:
$\mathrm{dM}=\mathrm{PRT}$
Rearranging the above equation, we get M=dRTP
The above equation gives the relation between the density and molar mass of a gaseous substance
Some Solved Examples
Example 1: A refrigeration tank holding 5 liters of gas with molecular formula C2Cl2 F4 at 298 K and 3 atm pressure developed a leak. When the leak was discovered and repaired, the tank had lost 76 g of the gas. What was the pressure of the gas remaining in the tank at 298 K?
1) 0.83
2)1.23
3)0.67
4)2.23
Solution
First, understand the question.
there is a tank holding 5 liter of gas with molecular formula C2Cl2 F4 at 298K at pressure 3 atm
after leaking 76 g of gas lost
We have to find the pressure of the gas remaining in the tank at 298K.
Now,
Given some gas is lost in grams, so we need to find the initial quantity in grams from PV=nRT and n = weight/molar mass
Then we will have the quantity in grams of remaining gas.
Then we have to convert it into moles and find p from PV=nRT.
According to the ideal gas equation, we have:
pV = nRT
Thus, n=3×50.082×298=0.613moles
Thus, the total weight of the gas originally present = 0.613 x 171g
= 105g
Now, 76g of the gas is already lost; thus, the remaining gas:
= 105 - 76 = 29g
Thus, total moles of the gas remaining = 29/171 = 0.17 moles
Again, according to the ideal gas equation, we have:
PV = nRT
Thus,$\mathrm{P}=0.17 \times 0.082 \times 2985=0.83 \mathrm{~atm}$
Hence, the correct answer is option (1)
Example 2: Two identical flasks contain gases A and B at the same temperature. If the density of A=3 g/dm3 and that of B=1.5 g/dm3, and the molar mass of A = 12 and B, the ratio of pressure exerted by gases is :
1)PAPB=2
2)PAPB=1
3) PAPB=4
4)PAPB=3
Solution
As we learned in the Ideal Gas Law in terms of density -
PM=dRT
- wherein
where
d - density of gas
P - Pressure
R - Gas Constant
T - Temperature
M - Molar Mass
PA=3RTMA
PB=1.5RTMB
PAPB=2MBMA=2×2MAMA= 4
Example 3: Which of the following statements is correct?
(a) Equal masses of all gases occupy the same volume at 57 P.
(b) An wat ideal gas cannot be liquefied.
(c) the kinetic energy of gaseous molecules is servo at .
(d) Gases having very low critical temperatures often show nan ideal behavior at room temperature.
1) a and c only
2) b and c only
3) a and d only
4) (correct) b and d only
Solution
(a) It is not equal masses but equal moles of g axes.
(b) An ideal gas does not exhibit molecular attractions.
(c) KE is zero at 0 K and not at $0^{\circ} \mathrm{C}$
(d) The room temperature is much larger than the critical temperature. Options (b) and (d) are correct.
Hence, the answer is option (4).
Example 4: An open vessel at $27^{\circ} \mathrm{C}$ is heated until two fifth of the ain in it has escaped from the vessel. Assuming that the volume of the Vessel remains constant, the temperature at which the vessel has been heated in
1) 700 K
2) (correct) 500 K
3) 750 K
4) $500^{\circ} \mathrm{C}$
Solution
given, temperature $\left(\mathrm{T}_1\right)=27^{\circ} \mathrm{C}=273+27=300 \mathrm{~K}$
volume of vessel = constant
press cure in vessel $=$ constant
the volume of ain reduced by $\frac{2}{5}$ so the remaining volume of ain is $\frac{3}{5}$. Let, at $T_1$ the volume of air inside the vessel be $n$ so at $T_2$ the volume of air will be $\frac{3}{5} n$.
Now, a s p and vane constant, 10
$\mathrm{n} \cdot \mathrm{~T}_1=\frac{3}{5} \mathrm{nT}_2-(1)$
Putting the value of $\mathrm{T}_1$ in equation (1) we get,
$\begin{aligned}
& \mathrm{n}=300=\frac{3}{5} \mathrm{n} \cdot \mathrm{~T}_2 \\
& \Rightarrow \mathrm{~T}_2=300 \times \frac{5}{3}=500 \mathrm{k}
\end{aligned}$
Hence, the answer is option (2).
Example 5: A 10 mg effervescent tablet containing sodium bicarbonate and oxalic acid releases 0.25 ml of at and ban Sf molar volume of is 250 L under such conditions, what is the percentage of sodium bicarbonate in each tablet?
1) (correct) 8.4
2) 0.84
3) 16.8
4) 33.6
Solution
$\begin{aligned}
& 2 \mathrm{NaHCO}_3+\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4 \longrightarrow 2 \mathrm{CO}_2+\mathrm{Na}_2 \mathrm{C}_4 \mathrm{O}_4+\mathrm{H}_2 \mathrm{O} \\
& 2 \mathrm{~mol} \\
& 1 \mathrm{~mol}
\end{aligned}$
⇒ In the reaction, several moles of $\mathrm{CO}_2$ produced.
$\begin{aligned}
\mathrm{n} & =\frac{\mathrm{PV}}{\mathrm{RT}}=\frac{1 \mathrm{bar} \times 0.25 \times 10^{-3} \mathrm{~L}}{0.082 \mathrm{LatmK}^{-1} \mathrm{~mol}^{-1} \times 298.15 \mathrm{~K}} \\
& =1.08 \times 10^{-5} \mathrm{~mol}
\end{aligned}$
Number of moles of $\mathrm{NaHCO}_3=\frac{\text { Wt.ofNaHCO }}{3}$
Molecular mass of $\mathrm{NaHCO}_3$
Hence, the answer is option (1).
Practice more questions with the link given below
Frequently Asked Questions (FAQs)
The gas particles, which have a very small volume. We can state that the gas particles are of similar size and that there are no intermolecular forces of attraction or repulsion between them. The gas particles we've seen move at random, as predicted by Newton's law of motion. Gas particles that collide with each other in perfect elastic collisions with no energy loss.
PV = nRT is an ideal gas equation law that links the macroscopic properties of ideal gas equations. We learned that an ideal gas equation is one in which particles do not attract or repel one another, take up no space, and have no volume.
We can claim that adjusting the volume of a gas at one temperature to its volume at another temperature is an example. T1/T2, which is always in Kelvin, is used to determine volume at the new temperature T2. As the mass remains constant, the new density at T2 is also constant.
Ideal gas equation does not exist in reality. It's a hypothetical gas that's been proposed to make the computations easier. The gas molecules in an ideal gas equation travel freely in all directions, and collisions between them are considered fully elastic, implying that no kinetic energy is lost as a result of the collision.
The ideal gas equation equation, however, has a number of drawbacks.
As long as the density is kept low, this equation holds.
This equation can be used for a single gas or a mixture of many gases, where ‘n' represents the total number of moles of gas particles in the mixture.
The Equation of States of an Ideal gas equation explains the easy relationship between relatively generic and accurate quantities or properties. Equation of States is a broad term for an equation that relates the relationship between P, V, and T of an ideal gas equation. The Equation of States is a term that refers to a relationship including various parameters of a material at equilibrium condition.
