Diagonal Relationship of B and Si
Diagonal relationship in the periodic according to _userdata, relates to the similarity between elements the diagonally adjacent in the periodic table. Boron and Silicon are metalloids, one in Group 13, while the other one is in Group 14. That is the kind of relationship that does exist between them. It occurs because when we go diagonally through the periodic table, the increase of atomic number is balanced by changes in atomic size and electronegativity so that similar chemical properties come to be observed. For example, Boron and Silicon can both have covalent bonding and have nearly the same electronegativity and ionization energy.
- Boron exhibits resemblance with its diagonal element silicon of group 14:
- Development of the Concept on Different Aspects
- Relevance/Application
- Some Solved Examples
- Summary
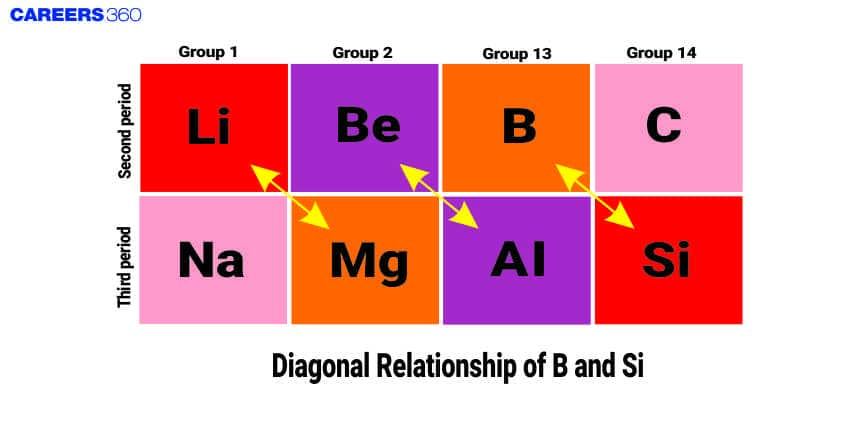
Boron exhibits resemblance with its diagonal element silicon of group 14:
- Both boron and silicon are non-metals.
- Both are semiconductors.
- Both form covalent hydrides i.e, boranes and silanes.
- Both form solid oxides that dissolve in alkalies forming borates and silicates respectively.
Development of the Concept on Different Aspects
Chemically, Boron and Silicon share a lot of features in common. These elements combine to form compounds that have the same structures. For example, boron forms borates, whereas silicon forms silicates; both compounds are the major constituents of glass and ceramics. These two members also form tetrahedral structured compounds in borates and silicates. In addition, Boron and Silicon are semiconductors; therefore, they have contributed a lot to the electronic industry. In this case, Boron is a doping agent in increasing the conductivity of semiconductors, while Silicon creates the spine in which most electronic devices are manufactured in the semiconductor industry. These parallels are further driven and underscored by the importance of their diagonal relationship in the different applications of industry.
Relevance/Application
The diagonal from Boron to Silicon is a phenomenally influential factor, both theoretically and in practice. This relationship will hence be very important in materials science since it can be used in the development of new material structures that have the desired properties. For example, borosilicate glass forms material that is very resistant to thermal and chemical attacks due to the pairing of boron and silicon; hence, it finds wideness of application in glassworks in laboratories and cookware. In electronics, their semi-conductor qualities are applied in the development of more efficient and small-sized electronic components. Another very important material, used in the making of most devices, is transistors and integrated circuits, which are the basics of most modern electronic devices; they would be the case for Boron-doped Silicon wafers. This knowledge is therefore a way of improving upon empirical chemical bonding and structure of molecules to represent very useful information in advancing many scientific fields.
Recommended topic video on(Diagonal relationship of B and Si)
Some Solved Examples
Example 1
Question: Which of the following elements does not show a diagonal relationship?
- B – Si
- Li – Mg
- Be – Al
- Li – Na
Solution:
Li–Mg, B–Si, and Be–Al show a diagonal relationship but Li and Na do not show a diagonal relationship as both belong to the same group and are not placed diagonally.
Hence, the correct answer is option (4).
Example 2
Question:
Which of the following shows a diagonal relationship with B?
- C
- Si
- Mg
- P
Solution:
Si shows a diagonal relationship with B due to their similar physical and chemical properties.
Therefore, the correct answer is option (2).
Example 3
Question: Which of the following pairs of elements form acidic oxides?
- B and Fe
- B and Si
- Mg and Si
- B and Mg
Solution:
Oxides of B and Si are both acidic and can be reduced by a limited amount of Mg.
Both are readily soluble in alkalies:
B2O3+3Mg→3MgO+2B
SiO2+2Mg→2MgO+Si
Hence, the correct answer is option (2).
Summary
The diagonal relationship of Boron and Silicon is a vivid image of the intricate and curious connections between elements in the periodic table. The parallelism defined by similar chemical behavior due to its diagonal position makes this relationship, thus, not only an academic concept but an affecting feature in many ways of application to industry requirements. The similarities in the behavior of Boron and Silicon, right through from the enhancement of the properties of materials to powering advances in the world of electronics, are a pointer to the understanding of periodic trends—in this way, we will delve into the behavior of elements and open avenues for potential scientific and technological advancement.
Frequently Asked Questions (FAQs)
When some elements on the periodic table that happen to be diagonally adjacent to each other exhibit similar properties, then there is a diagonal relationship. This results from the balancing of atomic size versus electronegativity across different periods and groups coming out to be similar, thus causing similar chemical properties.
Their diagonal relation could be explained by the fact that Boron and Silicon exhibit electronegativities, ionization energies, and a possibility of forming covalent bonds of similar magnitude. All these chemical properties result from their diagonal position in the periodic table.
Examples include emissions in the same structure, such as borates B2O3 and silicates SiO2, common in glass and ceramics. These elements exist in tetrahedral structures within their compounds.
Both Boron and silicon are semiconductors such that in industry, boron dopes silicon, a process that increases the semiconductor's conductance, and it acts as the basis for making transistors, and hence, eventually computers, which form part of most modern electronics
Also Read
19 Feb'25 06:55 PM
19 Feb'25 06:49 PM
19 Feb'25 06:45 PM
19 Feb'25 06:41 PM
19 Feb'25 05:03 PM
17 Oct'24 12:24 PM
11 Oct'24 10:25 AM
11 Oct'24 12:09 AM
05 Oct'24 12:20 AM

