Difference Between Cell and Battery - Types, FAQs
This article discusses the difference between cell and battery, the difference between cell padding and cell spacing, the function of cell and battery, difference between fuel cell and battery.
Batteries are the source of electric current that is used to start an automobile. small appliances like watches, clocks, radios, and other electric machines. A group of electrochemical cells which is a device for interconverting chemical and electrical energy is called a battery. In a spontaneous chemical reaction, the energy is released which is taken by batteries to form electricity.
- Types of Battery
- What is the difference between a cell and a battery?
- Working principle of galvanic cell
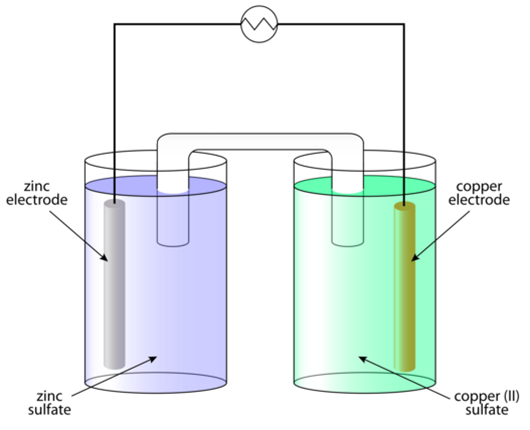 Figure 1 Cell
Figure 1 Cell
Types of Battery
Primary battery
Here, in these cells, electrode reaction is not reversed by flowing an external electrical current and reaction happens only once and after the completion of the reaction. It has become used and dead.
For example Dry cell and mercury cell
Secondary battery
Here, in these cells, electrode reactions can be reversed by flowing an external electrical current and hence can be recharged and used again and again.
For example Lead-acid storage cell and Ni-Cd cell.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is the difference between a cell and a battery?
Cell and battery difference is given below.
Cell | Battery |
It is a device that carries out the interconversion of ions between chemical energy and electrical energy | A battery is a group of electrochemical cells, a device for interconverting chemical and electrical energy. |
| It is light and compact because it is a single unit. | It is bulky and heavy as it is a combination of cells |
| The power supply of cell is for a short duration | The power supply of batteries is for long hours. |
| It is used in devices that require less energy such as lamps, radio, remote control devices, etc. | It is mostly used in inverters, heavy automobiles, construction, electric appliances, and many more. |
| Cost-wise it is cheap | Whereas batteries are costly. |
They are classified on the basis of electrolytes used in the column.
| They are classified into two types which are primary battery and secondary battery.
|
| A single electrochemical unit means one anode and one cathode and the electrolyte. | It is a collection of cells in series or parallel. |
Selection of Electrodes
Electrodes are always classified on the basis of process and are never classified on the basis of charge or polarity. It is emphasized on the fact that greater the reduction potential, reduction takes place on that electrode, and that respective electrode acts as the cathode and vice versa independent of the nature of the cell under consideration.
Anode Negative Loss of electron or oxidation takes place
Cathode Positive Gain of electron or reduction takes place
Galvanic cells consist of two half cells that are anodic and cathodic and the cell reaction is of redox type. In a voltaic cell, oxidation takes place at the anode and reduction at the cathode in the cell. This is represented in Figure 1 which shows that the zinc rod is treated as an anode that is immersed in ZnSO4 solution and the copper rod is treated as a cathode that is immersed in CuSO4 solution.
Related Topics, |
Working principle of galvanic cell
In a galvanic cell, the anode and cathode metal strip is dipped in electrolyte and the oxidation and reduction reaction takes place simultaneously. In a galvanic cell, there is a voltage difference between the electrolyte and metal. When the two metals are joined with the wire, the current flow due to the potential difference between metal and electrolyte in an electrochemical cell.
Anode
The reactive metal zinc is treated as an anode that is dipped in a salt solution of zinc sulphate. The zinc atom will move in the salt solution to form zinc ion Zn2+.
The reaction is shown below.
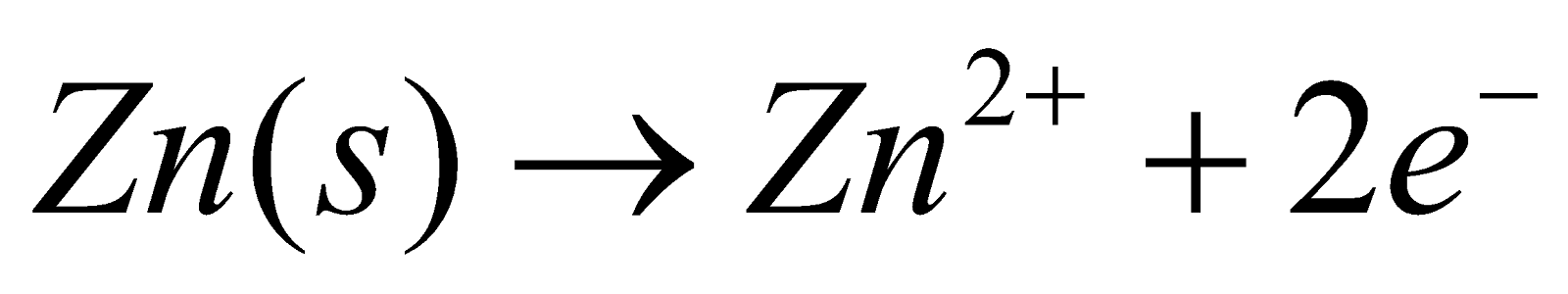
This will not allow the extra zinc ion to go in the solution.
The positive charge will move near the rod and the extra positive charge of the solution will move around the negative potential difference formed between the electrode and the electrolyte. In anode oxidation reaction takes place. During oxidation reaction electrons are released.
Cathode: Some metals like (Cu, Ag. Au etc.,) and many more are found to have the opposite propensity when placed in contact with their aqueous ions then the ions from the solution will get deposited on the metal rod in the electrochemical cell.
The following equilibrium will be established .
![]()
So the rod will have deficiency of electron (positive charge). The extra negative charge will move around the positively charged rod and form a double layer. This electrical double layer is established and the potential difference is formed between the rod and the solution known as electrode potential. At cathode, reduction occurs. In reduction, gain of electrons take place.
Their electrode potential can be represented by ECu2+.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 3 Electrochemistry
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3 Electrochemistry
- NCERT notes Class 12 Chemistry Chapter 3 Electrochemistry
Oxidation half reaction:
![]() (loss of electrons)
(loss of electrons)
Reduction half reaction:
![]() (gain of electrons)
(gain of electrons)
Overall reaction:
![]()
Salt Bridge:
The electrolyte in the salt bridge should be such that the speed of its cation equals the speed of its anion in the electric field. The salt bridge connects the solution of the two half cells by completing the circuit. The salt bridge helps to maintain the electric neutrality of the solution to form a continuous flow of current. Mainly KNO3 or NH4 NO3 is used to form salt bridge. The removal of salt bridge leads to voltage drop to zero.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Cell padding provides space within the cells or columns and cell spacing provides space outside the cell or columns.
Battery consists of several anodes and cathodes with electrolyte or it is an arrangement of many electrochemical cells in series or parallel to provide energy.
It is an electric battery or electrochemical cell. Electrochemical cells are of two types Galvanic cell and Electrolytic cell.
Galvanic cell/voltaic cell: It contains a spontaneous chemical reaction which generates an electric current.
Electrolytic cell: Here, an electric current drives a non spontaneous reaction.
Here, reactants, the products and electrolytes are all constantly passing through the cell and converting chemical energy into electrical energy. For example H2 and O2 fuel cells.
Mostly, lead storage cells are used in automobiles and inverters.
Also Read
02 Jul'25 05:05 PM
02 Jul'25 05:03 PM
02 Jul'25 05:03 PM
02 Jul'25 04:57 PM
02 Jul'25 04:51 PM
02 Jul'25 04:41 PM
02 Jul'25 04:39 PM
02 Jul'25 04:36 PM
02 Jul'25 04:32 PM