Difference Between Electrophile and Nucleophile - Definition, Examples, FAQs
Difference Between Electrophile and Nucleophile.
Electrophiles and nucleophiles are the most common substrates for chemical reactions between organic and inorganic chemical species. Derivatives of atoms or molecules are known as electrophiles and nucleophiles. Christopher Kelk Ingold invented these two concepts in 1933 to replace the terms cationoid and anionoid, both of which were initially coined by A.J. Lapworth in 1925. In general, a nucleophile is an electron donor, while an electrophile is an electron acceptor.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Difference Between Electrophile and Nucleophile.
- What are Electrophiles and Nucleophiles?
- Difference Between Electrophile and Nucleophile.
- Difference between nucleophile and base
- Difference between nucleophilicity and basicity.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What are Electrophiles and Nucleophiles?
Electrophile
The term "electrophile" comes from the Latin word "phile," which means "loving." Simply put, it means " electrons loving." It's a reagent with a low electron density in its valance shell that forms a covalent connection with a high-density molecule, ion, or atom. Electrophilic compounds include hydrogen ions found in acids and methyl-carbocation. They do not have enough electrons. An electrophile can easily be identified by a positive charge or a neutral charge with empty orbitals (not satisfying the octet rule).
Electrons go from a high-density area to a low-density area, and opposite charges attract one other. This hypothesis explains the attraction of electrons by electron-deficient electrophile atoms, molecules, or ions. An electrophile is interchangeably referred to as a Lewis acid since it accepts electrons according to the definition of the acid. Electrophiles are demonstrated in the reactions and compounds below. The hydroxide ion reacts with hydrogen chloride in this reaction, resulting in an acid-base reaction.
The more electronegative oxygen atom contributes electrons to the electron-deficient hydrogen atom, as shown by the arrow. Because it is more electronegative than hydrogen, it shares a lone pair with the hydrogen atom, which has a positive charge in the molecule hydrogen chloride. Different Lewis acid reaction and Lewis base reaction are based on this reaction.
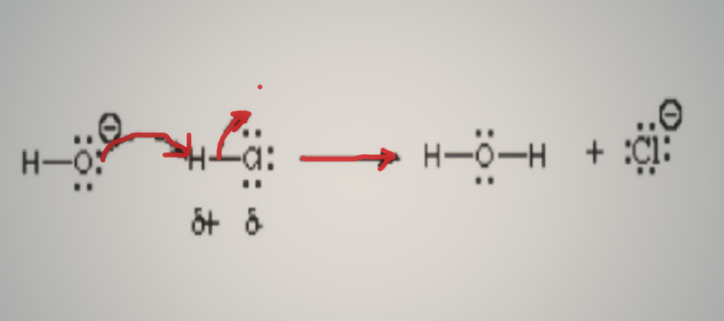
There is a partial positive charge in hydrogen chloride and a formal positive charge in methyl carbocation. These are examples of electrophile. Electrophiles includes polarised neutral molecules like acyl halides, carbonyl compounds, and alkyl halides. The full unoccupied orbitals in the outer shell of the hydrogen ion prevent it from being categorized as an electrophile, despite its positive charge. It produces hydrogen ion and water as a byproduct. The ammonium ion is the same way; it lacks empty orbitals that can grab electrons. As a result, it cannot be classified as an electrophile.
Eg :
- Chlorine ion, or Cl+, is an electrophile in its ionic form.
- Hydrogen ion
- Borane, or Boron Trihydride ( BH3), has an empty p orbital. As a result, it has the ability to attract electrons. As a result, it's an electrophile.
- AlCl3 is a fascinating substance. The compound's Cl atoms have entire octets. However, the valence shell of Al does not have eight electrons. As a result, it is drawn to electron-rich substances.
Nucleophile
The phrase is made up of two words: "nucleo," which refers to the nucleus, and "phile," which means "love." It simply means "loving the nucleus." Nucleophiles have a lot of electrons, thus they contribute electron pairs to electrophiles in chemical reactions to create covalent bonds. Lone pairs, pi bonds, and negative charges are the most noticeable of these compounds. Nucleophile compounds include ammonia, iodide, and hydroxide ions. Because they all donate electrons and receive protons, a nucleophile is also referred to as the Lewis base.
The most electronegative atom in a molecule is used to determine the nucleophilic core. Consider the nitrogen in ammonia (NH3), which is more electronegative and hence attracts electrons to the centre. When it reacts with an electrophile, such as water, the compound has a high electron density and donates electrons. Depending on the substance or molecule it reacts with, H2O can serve as both an electrophile and a nucleophile.
Consider the illustration below.
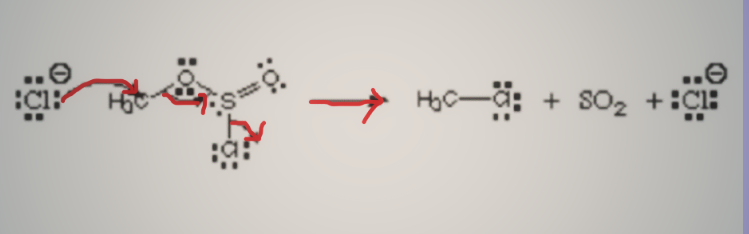
The first atom, the chloride ion, is forming a covalent bond with carbon by giving its lone pair. It is referred to as the nucleophile since it has a negative charge and donates electrons. The departing group refers to the chlorine atom that is exiting the chlorosulfite ester. It's not a nucleophile or an electrophile.
Eg:
- In its atomic form, chlorine (Cl) possesses three lone pairs of electrons. As a result, by becoming linked to other electron-deficient atoms or molecules, it can donate them to them.
- Because of its electronegativity, OH- can be a powerful nucleophile.
- A lone pair of electrons exists in NH3. As a result, it is a nucleophile.
| Related topics link, |
Difference Between Electrophile and Nucleophile.
- Electrophiles are classified as electron-loving species, whereas Nucleophiles are known as electron-donor species.
- Nucleophile contains negatively and neutrally charged atoms, ions, and electrons, whereas Electrophile contains positively and neutrally charged atoms, ions, and electrons.
- The Electrophile is an atom or molecule that may freely get a pair of electrons from electron-rich species such as an atom, ion, or molecule; the Nucleophile, on the other hand, is a molecule, an ion, or an atom that has a high density of electrons and can freely give a pair of electrons.
- Electrophile undertakes electrophilic addition reactions and electrophilic substitution reactions; Nucleophile, on the other hand, undergoes nucleophilic addition reactions and nucleophilic substitution reactions.
- Electrophiles are also known as Lewis acids because they quickly take electrons. The Nucleophile, on the other hand, quickly gives electrons to other species, which is why it is also known as Lewis bases.
- The Electrophile can be identified by a formal positive charge, a partial positive charge, or a neutral ion, atom, or molecule (that does not follow the octet rule); on the other hand, the Nucleophile can be identified by free electrons and positive charges (present in nucleophilic orbitals).
- Carbocations are the most common electrophiles. All Nucleophiles are referred to as carbanions in this comparison.
Electrophiles are denoted by the letter E, while nucleophiles are denoted by the letter NU-.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
Difference between nucleophile and base
Nucleophile
- Nucleophile attack the electron deficient carbons.
- Nucleophile are affected by electricity and speed.
- Nucleophile have lower negative charge.
Base
- Base attack acidic protons.
- Bases are affected by temperature.
- Bases have lower electronegativity charge.
Difference between nucleophilicity and basicity.
Basicity: Here, nucleophile attack the hydrogen.
Nucleophilicity: Here, nucleophile attack other atom except hydrogen.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
When a nucleophile attracts the proton of Hydrogen, it becomes a base. The essential function of a base is to attract hydrogen atoms. However, keep in mind that bases are only attracted to hydrogen ions and form connections with them. Nucleophiles, on the other hand, can attack any electron-deficient species. Nucleophiles are all bases, but bases aren't all nucleophiles
A nucleophile is a creature with a lot of electrons. The combination as a whole can be neutral, but individual atoms can have lone pairs of electrons, therefore it can be neutral. As a result, while being neutral, they can act as nucleophiles. The entire complex is attracted to the positive region of another molecule because of this lone pair of electrons.
Electrophiles are electron-poor organisms that can accept electron pairs from electron-rich organisms. The two examples are carbocations and carbonyl compounds. A nucleophile is an electron rich species that have the ability to donate electron pair to electron deficient species. Examples of nucleophile are carbanions, ammonia, cyanide ion.
Because the creation of the atoms makes it harder for the nucleophile to approach, BF4 is not a nucleophile. This is known as steric hindrance.
CO2 is an electrophile, to be sure. The electrons are drawn to the side of the two strong electronegative Oxygen atoms. As a result, the central carbon atom receives a partial + charge.
Also Read
12 Dec'24 04:49 PM
04 Nov'24 05:31 PM
21 Oct'24 05:53 PM
09 Oct'24 06:12 PM
09 Oct'24 04:00 PM
30 Sep'24 10:57 AM
30 Sep'24 08:52 AM