Difference Between Vapor and Gas - Properties, FAQs
To minimize the confusion, it's critical to understand the major differences between vapour and gas before using either term. Solid, Liquid, and Gas are the three states in which matter can be found to exist. Solids are predefined in terms of their shape and volume, unlike liquids (for example Ice). Water and other liquids have a specific volume, but depending on the container, they can take on different shapes.
- Vapour
- Measuring Vapour
- Examples of Gases and Vapour
- Gases
- Properties of Gases
The gaseous state is the third and final state, and it has neither a definite shape nor volume. Water vapour and oxygen are examples of gases. Matter can be transformed from one state to another by adjusting the temperature and pressure. When you lower the temperature or increase the pressure on a substance, the molecules get closer together and pack in more tightly.
This procedure permits the phase shifts from gas to liquids and from liquids to solid. The molecules move apart as the temperature and pressure are raised and lowered, and this can lead to phase transitions. from solids to liquids and also from liquids to gases. When normal conditions change, a material can skip a phase and proceed directly to the next.
Sublimation is the process by which a solid can be transformed directly into a gas without passing through an intermediate liquid stage. Dry ice, ammonium chloride, camphor, and iodine are a few examples of these substances. This article clears the questions like what is vapour, is water vapour a gas, what is the difference between gas and vapour etc.
Also, check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Vapour
Do you know what is meaning of vaporous? Let's look at what is vapour. Boiling a liquid produces vapour, which is created by a process called evaporation. Vapour definition is given as a state between the liquid and gas phases. When a liquid or solid is at equilibrium with it, it can coexist. Examples include water vapour and mercury vapour. Boiling or evaporation creates water in a gaseous state, which is referred to as water vapour.
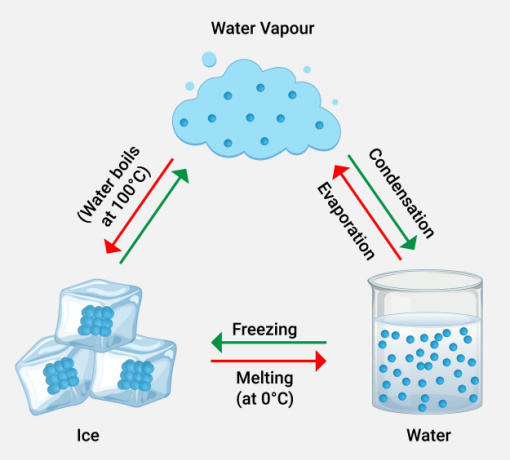
An aerosol is a mixture of gas and solid or liquid particles suspended in the gas. Water vapour has a greater greenhouse effect because it emits and absorbs infrared radiation over a wider range of wavelengths. In contrast to other greenhouse gases, water vapour makes up a disproportionately big portion of the earth's atmosphere. The amount of water vapour in the atmosphere changes constantly, making it impossible to get an accurate reading.
Measuring Vapour
Because it is in the gaseous state the partial pressure of the gases is used to calculate the amount of vapour present. In a gravitational field, vapours like normal atmospheric gases follow the Barometer Formula.
Examples of Gases and Vapour
As we know the most common example of vapour is water vapour (gas phase water) a room temperature and one atmosphere of pressure. And the second is gas; we know the most common example of gas is air (the air we breathe is gas). It can also be considered a mixture of many gases, such as oxygen, nitrogen, carbon dioxide, etc.
Gases
Gas is one of the four states of matter, and one of its distinguishing characteristics is that it takes up all of the available space, independent of its shape or volume. This property is due to the molecules having relatively low intermolecular attraction. Solid, liquid, and plasma are the other states of matter.
Solids have a fixed shape and volume, while liquids have a fixed volume but lack the fixed shape attribute; they take on the shape of the container they are poured into. Due to the weak interaction between the molecules, the gaseous molecules move constantly and independently. Due to the continual motion of the gaseous molecules, the gas can fill any size container. Compounds are categorised as gases even if they are still in a gaseous state. Carbon dioxide, for example, is a gas because it continues to be a gas at room temperature.
It is possible to create compressible fluid by compressing a gas's molecules, which are constantly moving past one another. Fixed gases are those gases that, regardless of temperature, cannot solidify or liquid is. Gases can't be seen with the human eye since their molecules are so far apart. At room temperature, gases such as carbon dioxide exist in gaseous form.
Also, students can refer to,
- NCERT Solutions for Class 11 Chemistry Chapter 5 States of Matter
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 States of Matter
- NCERT Notes Class 11 Chemistry Chapter 5 States of Matter
Properties of Gases
They're easily compacted, which makes them useful. Intermolecular attraction keeps gas molecules apart in a steady state. Because of this, gases can be compressed with little effort, and this property is employed to compress gases in science, medicine, and engineering. The increased pressure and decreased temperature are used in compression.
Spray bottles, which compress the gases inside to make it easier to spray perfume when needed, are an excellent illustration of how air compression is used. No matter how big or small they are, they may all fit into the container. When you fill a balloon with air, the molecules are distributed evenly across the container. Gravity, on the other hand, limits the amount of liquid that may be put into a container.
Liquids in containers are shaped by gravity, whereas gases are less thick and can spread evenly. They take up more room than either solids or liquids. Oxygen and nitrogen are the most prevalent gases in the atmosphere (78 percent Nitrogen and 21 percent Oxygen). It contains a wide range of different gases, all in quite small concentrations. Difference between gases and vapors/ difference between steam and vapour:
Related Topics Link, |
What is the Difference Between Gas And Vapour?
For the most part, a vapour phase is made up of two separate substances, one at ambient temperature and the other at a specific thermodynamic range. As a result, these are the primary differences between Vapor and Gas. This is the main difference between gas and vapour.
Vapours are usually solid or liquid, but they can become gaseous when exposed to certain conditions. It's not a physical property. Under normal circumstances, gases are in a gaseous state (at room temperature and one atmospheric pressure). A gaseous state of matter is one in which nothing exists. This is another difference between vapour and gas.
Whenever a vapour goes from liquid to vapour or from solid to vapour, it undergoes a phase change. The phase of a gas does not change. Any phase shift transforms a gas into a liquid. This is also a difference between gas and vapour.
The natural state of vapour is not gaseous, but might be solid or liquid. But the natural form of gas is in the gaseous state. This is another important difference between gas and vapour.
Under normal circumstances, the vapour is a substance that is both gaseous and liquid in nature. In normal circumstances, gas has a single thermodynamic state. This is another difference between gas and vapour.
The molecules and atoms that make up vapour move at random. Atoms and molecules in gases travel in random directions. This is also a difference between gas and vapour.
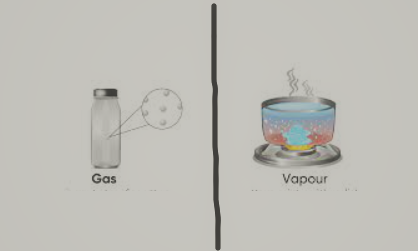
In this way, gas and vapour difference is made.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
At normal temperature, vapour is a mixture of two or more distinct phases: the liquid and gaseous phases. At room temperature, gas normally has a single thermodynamic condition.When vapour is examined under a microscope, it reveals a clump of particles with no discernible shape.When gas is viewed under a microscope, it lacks a distinct shape.The molecules and atoms in a vapour are a random collection of particles travelling about in space.The molecules and atoms that make up gas are also made up of unorganised chaos.Contrary to gases, vapour is not a state of substance. The state of matter is gaseous.We are constantly surrounded by water vapour, even when the temperature is below the boiling point.Most gases arise when a substance reaches a temperature or pressure that is greater than or equal to its critical value.
It is the gaseous state of water.
Under the critical temperature, a substance can exist in two states simultaneously: solid or liquid. This is called a vapour phase. When the vapour and either the solid or liquid phase are closely associated, both phases are in balance. The term "gas" often refers to a fluid phase that is easily compressed. Cloud formation is linked to vapour condensation. Vapor molecules have three types of motion: rotation, translation, and vibration.
When discussing vapour pressure, scientists refer to it as the pressure that solids or liquids exert when heated to a specific temperature. The vapour pressure is described by Raoult's law, which states that the partial pressure of any component is equal to the product of the pure component's vapour pressure and the mole fraction of the mixture.
It's the condition in which all of the particles are a long way apart. It's one of the three fundamental states of matter. When it comes to gases, the intermolecular distances between molecules are enormous and can be compressed with ease. It is impossible to measure the attractive forces that exist between the gas particles. The substances that exist as gaseous mixtures have neither a specific structure nor volume. They take up the entire volume if they're contained. They next apply a set amount of pressure to the container's walls.
Also Read
07 Jun'25 04:51 PM
11 Mar'25 12:08 PM
19 Feb'25 11:49 AM
19 Feb'25 11:23 AM
19 Feb'25 11:14 AM
19 Feb'25 10:31 AM
18 Feb'25 12:15 PM
18 Feb'25 11:59 AM
09 Feb'25 10:16 PM
08 Feb'25 05:31 PM

