Displacement reaction
The concept of displacement reactions, also known as single-replacement reactions or the double replacement at the same time, was developed over time through the contributions of several chemists rather than being discovered by a single individual. These reactions involve a more reactive element displacing a less reactive element from a compound. In the early 19th century, Antoine Lavoisier, a French chemist often considered the "father of modern chemistry," made significant contributions to our understanding of chemical reactions, including displacement reactions. Lavoisier's work on the nature of chemical reactions and the concept of elements and compounds laid the groundwork for further studies on displacement reactions.
In the mid-19th century, more detailed investigations into the nature of these reactions were conducted by chemists like Dmitri Mendeleev, who developed the periodic table, providing insights into the reactivity of elements. The periodic table helped explain why certain elements can displace others in reactions, based on their position and reactivity. The study of displacement reactions continued to evolve as the field of chemistry developed, with contributions from various scientists over time. The reason for studying these reactions is to understand how different elements interact and react with each other, which is crucial for both theoretical chemistry and practical applications in industries such as metallurgy and materials science.
Displacement Reaction
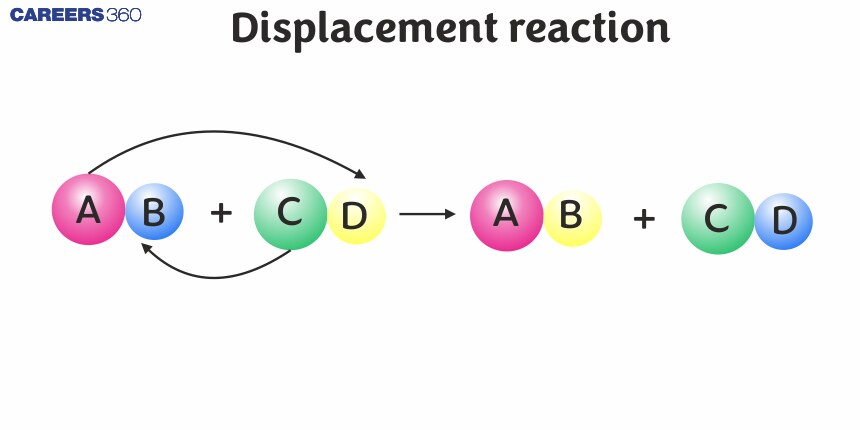
A displacement reaction, also known as a single replacement or single displacement reaction, is a type of chemical reaction where one element displaces another element in a compound. This reaction typically involves two reactants: a single element and a compound.
In a displacement reaction, an ion (or an atom) in a compound is replaced by an ion (or an atom) of another element. It may be denoted as:
$\mathrm{X}+\mathrm{YZ} \rightarrow \mathrm{XZ}+\mathrm{Y}$
Displacement reactions fit into two categories: metal displacement and non-metal displacement.
- Metal Displacement: A metal in a compound can be displaced by another metal in an uncombined state. Metal displacement reactions find many applications in metallurgical processes in which pure metals are obtained from their compounds in ores. A few such examples are:
$\begin{aligned} & \mathrm{CuSO}_4(\mathrm{aq})+\mathrm{Zn}(\mathrm{s}) \rightarrow \mathrm{Cu}(\mathrm{s})+\mathrm{ZnSO}_4(\mathrm{aq}) \\ & \mathrm{V}_2 \mathrm{O}_5(\mathrm{~s})+5 \mathrm{Ca}(\mathrm{s}) \xrightarrow{\Delta} 2 \mathrm{~V}(\mathrm{~s})+5 \mathrm{CaO}(\mathrm{s}) \\ & \mathrm{TiCl}_4(\mathrm{l})+2 \mathrm{Mg}(\mathrm{s}) \xrightarrow{\Delta} \mathrm{Ti}(\mathrm{s})+2 \mathrm{MgCl}_2(\mathrm{~s})\end{aligned}$
In each case, the reducing metal is a better reducing agent than the one that is being reduced which evidently shows more capability to lose electrons as compared to the one that is reduced.- Non-metal displacement: The non-metal displacement redox reactions include hydrogen displacement and a rarely occurring reaction involving oxygen displacement. All alkali metals and some alkaline earth metals (Ca, Sr, and Ba) which are very good reductants, will displace hydrogen from cold water.
- Less active metals such as magnesium and iron react with steam to produce dihydrogen gas:
$2 \mathrm{Na}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})$
$\mathrm{Ca}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Ca}(\mathrm{OH})_2(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})$
$\mathrm{Mg}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \xrightarrow{\Delta} \mathrm{Mg}(\mathrm{OH})_2(\mathrm{~s})+\mathrm{H}_2(\mathrm{~g})$
Recommende topic video on (Displacement reaction)
Some Solved Examples
Example.1
1. What would you classify the following reaction as?
$2 \mathrm{H}_2 \mathrm{O}_{(l)}+2 \mathrm{~F}_{2(g)} \rightarrow 4 H F_{(a q)}+O_{2(g)}$
1)Decomposition reaction
2)disproportionation reaction
3)metal displacement reaction
4) (correct)non-metal displacement reaction
Solution
Non-metal displacement: Redox reactions include hydrogen displacement and a rarely occurring reaction involving oxygen displacement.
$2 \mathrm{H}_2 \mathrm{O}_{(l)}+2 \mathrm{~F}_{2(g)} \rightarrow 4 H F_{(a q)}+O_{2(g)}$
Hence, the answer is the option (4)
Example.2
Match the following reactions to their types.
1.$2 \mathrm{Na}_{(s)}+2 \mathrm{H}_2 \mathrm{O}_{(t)} \rightarrow 2 \mathrm{NaOH}_{(a q)}+\mathrm{H}_{2(g)}$
p.disproportionate reaction
2.$2 \mathrm{NH}_{3(g)} \rightarrow \mathrm{N}_{2(g)}^{+}+3 \mathrm{H}_{2(g)}$
q.decomposition reaction
3.$V_2 O_{5(g)}+5 \mathrm{Ca}_{(\mathrm{s})} \xrightarrow{\Delta} 2 V_{(s)}+5 \mathrm{CaO}_{(s)}$
r.Non-Metal Displacement Reaction
4.$\mathrm{Cl}_{2(g)}+2 \mathrm{OH}_{(a q)}^{-} \rightarrow \mathrm{Cl}_{(a q)}^{-}+\mathrm{ClO}_{(a q)}^{-}+\mathrm{H}_2 \mathrm{O}_{(l)}$
s.Metal Displacement Reaction
1)1-q,2-p,3-s,4-r
2) (correct)1-r,2-q,3-s,4-p
3)1-s,2-p,3-q,4-r
4)1-r,2-q,3-p,4-s
Solution
Decomposition Reaction
This reaction involves the breakdown of a compound into different compounds.
$2 \mathrm{NH}_{3(g)} \rightarrow \mathrm{N}_{2(g)}^{+}+3 \mathrm{H}_{2(g)}$
Disproportionation Reactions
Disproportionation reactions are those reactions in which a single element in one oxidation state is simultaneously oxidized and reduced.
$\mathrm{Cl}_{2(g)}+2 \mathrm{OH}_{(a q)}^{-} \rightarrow \mathrm{Cl}_{(a q)}^{-}+\mathrm{ClO}_{(a q)}^{-}+\mathrm{H}_2 \mathrm{O}_{(l)}$
Metal Displacement: A metal in a compound can be displaced by another metal in an uncombined state.
$V_2 O_{5(g)}+5 \mathrm{Ca}_{(s)} \xrightarrow{\Delta} 2 V_{(s)}+5 \mathrm{CaO}_{(s)}$
Non-metal displacement: Redox reactions include hydrogen displacement and a rarely occurring reaction involving oxygen displacement.
$2 \mathrm{Na}_{(s)}+2 \mathrm{H}_2 \mathrm{O}_{(l)} \rightarrow 2 \mathrm{NaOH}_{(a q)}+\mathrm{H}_{2(g)}$
Correct Match : 1-r,2-q,3-s,4-p
Hence, the answer is the option (2).
Example.3
1. What would you classify the following reaction as
$\mathrm{PbCl}_2+\mathrm{Zn} \rightarrow \mathrm{ZnCl}_2+\mathrm{Pb}$
1) (correct)Metal displacement reaction
2)decomposition reaction
3)non-metal displacement reaction
4)disproportionation reaction
Solution
Displacement Reaction -
In a displacement reaction, an ion (or an atom) in a compound is replaced by an ion (or an atom) of another element. It may be denoted as:
$\mathrm{X}+\mathrm{YZ} \rightarrow \mathrm{XZ}+\mathrm{Y}$
Displacement reactions fit into two categories: metal displacement and non-metal displacement.
It's a metal displacement reaction since Zn replaces Pb from PbCl2 leaving lead in an elemental state.
Hence, the answer is the option (1).
Example.4
4. Match the following reactions to their types.
1.1. $\mathrm{CuCl}_2 \rightarrow \mathrm{Cu}+\mathrm{Cl}_2$
p.Metal Displacement Reaction
2.$3 A l_{(s)}+3 C u S O_{(a q)} \rightarrow 3 C u_{(s)}+A l_2\left(\mathrm{SO}_4\right)_3$
q.disproportionate reaction
3.$2 \mathrm{~K}+\mathrm{Cl}_2 \rightarrow 2 \mathrm{KCl}$
r.Combination reaction
4.$3 \mathrm{CrO}_4^{3-}+8 \mathrm{H}^{+} \rightarrow 2 \mathrm{CrO}_4^{2-}+\mathrm{Cr}^{3+}+4 \mathrm{H}_2 \mathrm{O}$
s.decomposition reaction
1) (correct)1-s,2-p,3-r,4-q
2)1-s,2-r,3-p,4-q
3)1-r,2-s,3-p,4-q
4)1-s,2-r,3-q,4-p
Solution
Decomposition Reaction
This reaction involves the breakdown of a compound into different compounds.
$\mathrm{CuCl}_2 \rightarrow \mathrm{Cu}+\mathrm{Cl}_2$
Combination Reaction
These reactions are the opposite of decomposition reactions and, hence, involve the combination of two species to form a single compound.
$2 \mathrm{~K}+\mathrm{Cl}_2 \rightarrow 2 \mathrm{KCl}$
Disproportionation Reactions
Disproportionation reactions are those reactions in which a single element in one oxidation state is simultaneously oxidized and reduced.
$3 \mathrm{CrO}_4^{3-}+8 \mathrm{H}^{+} \rightarrow 2 \mathrm{CrO}_4^{2-}+\mathrm{Cr}^{3+}+4 \mathrm{H}_2 \mathrm{O}$
Metal Displacement: A metal in a compound can be displaced by another metal in an uncombined state. Metal displacement reactions find many applications in metallurgical processes in which pure metals are obtained from their compounds in ores.
$2 \mathrm{Al}_{(\mathrm{s})}+3 \mathrm{CuSO}_{4(a q)} \rightarrow 3 \mathrm{Cu} u_{(s)}+\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$
Hence, the answer is the option (1).
Example.5
5. Which of the following can be classified as the Metal Displacement Reaction?
1)$\mathrm{Mg}+\frac{1}{2} \mathrm{O}_2 \rightarrow \mathrm{MgO}$
2) (correct)$\mathrm{CuSO}_4+\mathrm{Ca} \rightarrow \mathrm{CaSO}_4+\mathrm{Cu}$
3)$\mathrm{CaCO}_3 \rightarrow \mathrm{CaO}+\mathrm{CO}_2$
4)$\mathrm{Cu}_2 \mathrm{O}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Cu}+\mathrm{CuSO}_4+\mathrm{H}_2 \mathrm{O}$
Solution
Option(1) is the only combination reaction.
Option(2) is the only metal displacement reaction.
Option(3) is the Decomposition reaction.
Option(4) is a disproportionation reaction.
Hence, the answer is the option (2).
Summary
Displacement reactions have numerous benefits and practical applications in various fields. Such as Industrial Applications which include metal extraction displacement reactions are crucial in extracting metals from their ores. For example, in the extraction of zinc from zinc sulfate using a more reactive metal like aluminum. This method allows the recovery of valuable metals from their ores, which is essential for manufacturing and technological applications. In the production of Chemicals, displacement reactions are used in producing chemicals such as hydrogen chloride from hydrochloric acid and zinc. Enables the synthesis of various chemicals and reagents used in industry and laboratories.