Electrodes - Definition, Factors, Types, Uses, FAQs
What allows electrons to enter or leave an electrochemical system? How does a solid surface conform to the oxidation and reduction reactions in an electrochemical cell? An electrode is the conductor through which electric current enters or leaves an electrolyte, allowing redox reactions on its surface. The electrode assumes importance in electrogeneration and electrolysis for determining the direction of electron flow and the type of reaction.
This Story also Contains
- Electrodes
- Types of Electrodes
- Active Electrode
- Inert Electrode
- Cathode and Anode in Electrochemical Cells
- Factors Affecting Products of Electrolysis
- Uses of electrodes
- Some Solved Examples
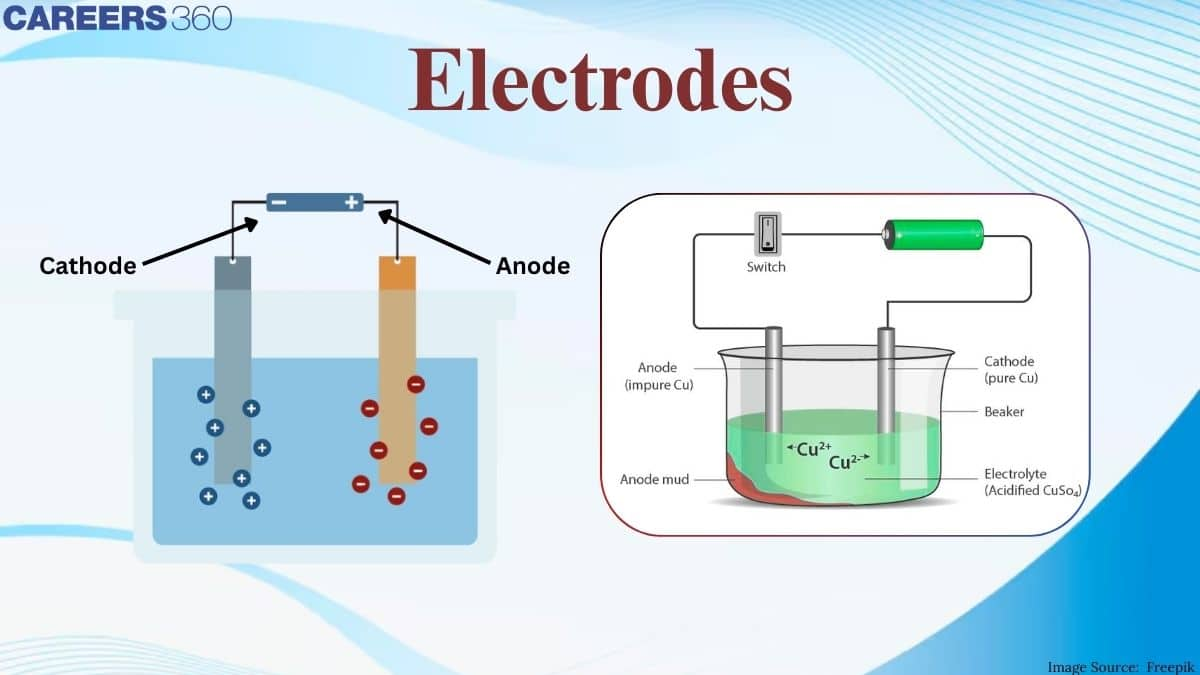
Electrodes
Electrode meaning is a point where current enters or leaves the electrolyte or circuit is referred to as an electrode. The cathode is where the current departs the electrode, while the anode is where the current enters the electrode. Electrodes are the basic building blocks of electrochemical cells. A good conductor of electricity is required for an electrode. Although there are inert electrodes that do not participate in the process. Gold, platinum, carbon, graphite, metal, and other materials can be used as electrodes. In the cells, the electrode provides a surface for oxidation-reduction reactions.
Types of Electrodes
There are two types of electrodes:
- Active electrodes
- Inert electrodes.
Active electrode materials are those that participate in the chemical process in the cell and can dissolve in the electrolyte. Copper electrodes, silver electrodes, zinc electrodes, copper electrodes, and so on are examples of reactive electrodes. These are mostly employed in potentiometric measurements.
Active Electrode
In electrochemical cells, the active electrode is a metal electrode. It takes part in the electrolyte's processes in order to transmit the power. It is possible to oxidize or decrease the active electrode. Electroplating is the most common application for active electrodes. Electroplating is the process of applying one metal to another metal using an electrochemical cell. A spoon, for example, can be silver-plated by employing a silver anode and the spoon as the cathode, with silver nitrate as the electrolyte. The active electrode gets its name from the fact that it actively participates in the chemical reaction that takes place in the system. It actively exchanges ions with an electrolytic solution as a result.
Also read :
Inert Electrode
A metal that does not participate in or interfere with any chemical reaction is known as an inert electrode. However, rather of exchanging ions with the solution, it is still employed to conduct electricity via transferring electrons. As a result, it functions as an electron. As an inert electrode, platinum is employed. However, graphite is commonly used because to its low cost. However, graphite is commonly used because to its low cost. In the process of conducting electricity, an inert electrode can provide or withdraw electrons. Electrolysis, the process of separating an ionic compound into its constituent elements, always uses inert electrodes. When sodium chloride solution is electrolyzed, sodium and chlorine are produced individually.
Cathode and Anode in Electrochemical Cells
An electrode in an electrochemical cell is referred to as either a cathode or an anode. The anode is the electrode where electrons leave the cell and oxidation takes place, whereas the cathode is the electrode where electrons enter the cell and reduction takes place. Depending on the direction of current through the cell, either of the two electrodes can become an anode or a cathode. Bipolar electrodes are those that can operate as an anode in one cell and a cathode in another.
Primary cells are electrochemical cells in which irreversible reactions occur, which is why the cathode and anode identities are fixed in these cells. The anode in these cells will always be negative, implying that oxidation will always occur. While the cathode will always be positive, or at this point, there will always be a reduction. Galvanic cell is an example of a primary cell. Secondary cells, also known as electrolytic cells, are rechargeable, which means they undergo reversible chemical reactions. The anode is always positive in these cells, while the cathode is constantly negative.
|
Related Topics Link |
Factors Affecting Products of Electrolysis
1. The electrolysis products are determined by the material being electrolyzed. In other words, the electrolysis process is governed by the type of the electrolyte. For a strong electrolyte, the procedure is quick, but for a weak electrolyte, an extra potential, also known as excess potential, is necessary. The value of this excess potential affects the electrolysis products as well.
2. The type of the electrodes has an impact on the electrolysis products. In other words, an inert electrode (such as gold or platinum) does not participate in the process, whereas an active electrode does.
3. The electrolytic products are affected by various oxidising and reducing species present in the electrolytic cell.
4. The electrolytic cell's products are determined by the standard electrode potentials of the various oxidising and reducing species present.
5. In the case of several reactions, the electrolysis product is determined by the standard electrode potential of the individual reactions. For instance, electrolysis of a sodium chloride aqueous solution. The reduction process with the highest standard electrode potential takes place at the cathode, among the many reduction reactions taking place. Similarly, the oxidation process with the lowest standard electrode potential occurs at the anode, among the many oxidation reactions.
Uses of electrodes
In a cell, electrodes are utilised to make contact between non-metal circuit components.
-
Conductivity is measured using electrodes.
-
These are utilised in car fuel cells.
-
Medical devices such as EEG, ECG, ECT, and defibrillators employ them.
-
These are employed in biomedical research for electrophysiological procedures.
-
These are employed in the electric chair execution.
-
Electroplating is done with them.
-
Arc welding is done with them.
-
These are utilised as a grounding device.
-
Electrochemistry makes use of them.
-
These are used to determine the chemical composition of substances.
-
These are utilised in the assembly of membrane electrodes.
-
Electroshock weapons take advantage of them
Also read -
Some Solved Examples
Question 1: In an electrochemical cell, the electrode at which oxidation occurs is called:
A. Cathode
B. Anode
C. Salt bridge
D. Electrolyte
Solution:
-
Oxidation always occurs at the anode (loss of electrons).
-
This rule is universal for both galvanic and electrolytic cells.
Hence, option B is correct.
Question 2: Which of the following statements is always correct for an electrode?
A. Anode is negatively charged
B. Cathode is positively charged
C. Oxidation occurs at anode
D. Reduction occurs at anode
Solution:
-
Charge on electrodes depends on cell type (galvanic or electrolytic).
-
But oxidation at anode and reduction at cathode are universal rules.
Hence, option C is correct.
Question 3: In a Daniell cell ( $\mathrm{Zn}\left|\mathrm{Zn}^{2+} \| \mathrm{Cu}^{2+}\right| \mathrm{Cu}$ ), the cathode is:
A. Zinc electrode
B. Copper electrode
C. Salt bridge
D. Both electrodes
Solution:
Zn undergoes oxidation → anode
$\mathrm{Cu}^{2+}$ gains electrons → reduction at cathode Thus, copper acts as cathode.
Hence, option B is correct.
Question 4: Which electrode is used as an inert electrode?
A. Copper
B. Zinc
C. Platinum
D. Sodium
Solution:
Inert electrodes do not participate in the reaction.
Common inert electrodes: Platinum (Pt) and Graphite (C)
Hence, option C is correct.
Frequently Asked Questions (FAQs)
1. The electrolysis products are determined by the material being electrolyzed. In other words, the electrolysis process is governed by the type of the electrolyte. For a strong electrolyte, the procedure is quick, but for a weak electrolyte, an extra potential, also known as excess potential, is necessary. The value of this excess potential affects the electrolysis products as well.
2. The type of the electrodes has an impact on the electrolysis products. In other words, an inert electrode (such as gold or platinum) does not participate in the process, whereas a active electrode does.
Active electrode | Inert electrode | |
Definition | The active electrode is the one that actively participates in the electrochemical cell's chemical reaction. | An inert electrode is one that is not involved in the chemical reaction. |
Uses | In electroplating, active electrodes are employed. | In electrolysis, inert electrodes are utilised. |
Behavior | The active electrode's metal ions dissolve in the electrolytic solution. | The inert electrode's metal ions are not dissolved. |
Reactions | On the active electrode, oxidation or reduction reactions may occur. | There are no reactions of oxidation or reduction. |
Mode of Electrical conductance | Ion exchange allows active electrodes to conduct electricity. | Electron transfer is used to carry electricity between inert electrodes. |
1. In a cell, electrodes are utilised to make contact between nonmetal circuit components.
2 .Conductivity is measured using electrodes.
3. These are utilised in car fuel cells.
4. Medical devices such as EEG, ECG, ECT, and defibrillators employ them.
Primary cells are electrochemical cells in which irreversible reactions occur, which is why the cathode and anode identities are fixed in these cells. The anode in these cells will always be negative, implying that oxidation will always occur. While the cathode will always be positive, or at this point, there will always be a reduction. The Galvanic cell is an example of a primary cell.
The point where current enters or leaves the electrolyte or circuit is referred to as an electrode.
Questions related to
On Question asked by student community
Correct Answer: Only 1,2 , and 4
Solution : The correcrt option is 4 i.e "Only 1, 2, and 4."
Explanation:
Let's refer to the following lines of the passage:
The device has a very simple structure consisting of specially designed nanocomposite polymers and contact electrodes and can generate a
Correct Answer: 1887
Solution : The correct option is 1887.
In the year 1887, German physicist Heinrich Hertz made a pivotal discovery related to the photoelectric effect while researching radio waves. In the course of his experiments, Hertz utilised a spark gap consisting of two closely spaced, sharp electrodes capable
Correct Answer: copper at the cathode, and oxygen at the anode
Solution : The correct option is copper at the cathode and oxygen at the anode.
Copper is deposited at the cathode and is dissolved at the anode. Consequently, the concentration of copper ions in the solution remains constant.
Correct Answer: Beryllium
Solution : The correct answer is Beryllium.
The symbol of Beryllium is Be with an atomic number of 4. It is a lightweight, metallic element known for its strong and lightweight properties. Beryllium is often used in alloys, especially in the aerospace industry, due to its