Electrons - Meaning, Definition, Properties, FAQs
Electrons are involved in a variety of physical processes, including electricity, magnetism, chemistry, and thermal conductivity, as well as gravitational, electromagnetic, and weak interactions. Because an electrons has charge, it has an electric field surrounding it, and if that electrons moves relative to an observer, the observer will notice it and generate a magnetic field. When electrons are accelerated, they emit or absorb energy in the form of photons. Electromagnetic fields can be used to capture individual electrons as well as electrons plasma in laboratory apparatus.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is electron definition?
- Discovery of electrons;
- Properties of electrons:
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
What is electron definition?
Electron Definition and electron meaning: The electron is a subatomic particle (with the electron symbol e- or β− ) with a negative one elementary electric charge. Electrons are the first generation of the lepton particle family, and because they have no known components or substructure, they are considered elementary particles. The charge held by an electron is the same as the charge held by a proton (but has an opposite sign). As a result, electrically neutral atoms/molecules must have the same number of protons and electrons. Although the magnitude of the charges borne by protons and electrons are the same, an electron’s size and mass are substantially smaller than a proton's (an electron’s mass is around 1/1836 that of a proton). Mass of electron is, like all elementary particles, have both particle and wave properties: they may collide with other particles and be diffracted like light.
Discovery of electrons;
During the 1880s and 1890s, scientists looked for the carrier of electrical qualities in materials using cathode rays. Their efforts culminated in the 1897 discovery of the electron atoms by English physicist J.J. Thomson.
The Cathode Ray Experiment by Thomson.
In chemistry, his electron atoms definition became known as the plum-pudding model, owing to the enormous number of electrons present in a form that formed an overall positive charge, thus generating an overall neutral charge. This model was superseded by the contemporary atomic model after the discovery of protons and neutrons.
Charge of electrons:
A negatively charged particle is an electron. The magnitude of the negative charge is
1.602 x 10-19 coulomb. An electrons has a mass of 1/1837 that of a proton.
Related Topics, |
The mass of an electron is:
An electrons has a mass of 9.10938356 x 10-31 kilograms. When compared to the mass of the proton, the electrons has a tiny mass.
Because of their small size of electron and mass, electrons' properties can be explored more thoroughly using quantum mechanics rather than classical mechanics. Because matter acts differently at the quantum scale, this is the case.
According to Heisenberg's uncertainty principle, the uncertainty associated with the position and velocity of an electrons is substantially greater than that associated with a proton or neutron. Atomic orbitals, which may be simply seen as zones surrounding the nucleus where the likelihood of finding a given electrons is high, are where electrons are distributed around the nuclei of atoms.
The helium atom
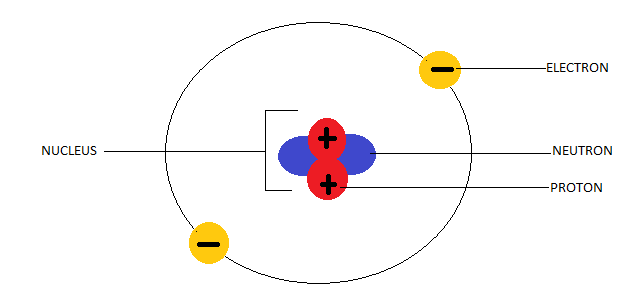
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 2 Structure of Atom
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 2 Structure of Atom
- NCERT notes Class 11 Chemistry Chapter 2 Structure of Atom
Properties of electrons:
Quantum particles have their own set of particular qualities that are not shared by other particles. Protons, neutrons, and electrons have well-defined properties for the most part. Here are some of an electrons's characteristics:
The mass of an electrons is 9.1 x 10-31 kg, which is equal to 0.000548579909 atomic mass units. An electrons's mass can also be expressed as 1/1840 of the mass of a hydrogen atom. Because the mass of a hydrogen atom is 1 u, the relative mass of an electrons can be written as 1/1840 u.
An electrons's absolute mass is 9 x 10-28 grams.
The effective charge of an electrons is -1 because the electrons is a negatively charged particle.
Because electrons have a negative charge, the electrical charge should be 1.602 x 10-19 coulombs.
Electrons orbit the nucleus of the atom in particular, well-defined orbits around the element's nucleus.
The properties of an electrons are unaffected by the gas present in the discharge tube and are independent of it.
Electrons also have a two-sided nature known as wave-particle duality, which implies that under certain conditions, the electrons, which is a particle, behaves like a wave.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Element atoms are neutral because a proton has a positive charge (+) and an electrons has a negative charge (-), with all positive charges cancelling out all negative charges. An atom's number of protons, neutrons, and electrons vary from one to another.
In 1891, G. Johnstone Stoney used the name "electrons" to represent the unit of charge discovered in experiments involving the transmission of electric current via chemicals. In this sense, J.J. Thomson's Cambridge classmate Joseph Larmor used the phrase.
The electrons was discovered as a result of JJ Thomson's experiment with cathode tubes. Cathode ray tubes are just glass vacuum chambers that are sealed. Thomson used two oppositely charged electric sites around the cathode ray to prove the existence of electrons. Thomson noticed that the cathode ray was redirected towards the negatively charged electric plate. As a result, the cathode ray was effectively proven to be made up of negatively charged particles.
In an atom, the quantities of protons and electrons are equal. Atoms are normally neutral because protons and electrons have equal and opposing charges.
When we have two electrons combined electric fields result in higher as well as higher potential energy since they get closer. In order to minimize the potential energy,they repel each other.
Also Read
06 Feb'25 11:21 PM
30 Dec'24 03:07 PM
20 Dec'24 11:57 PM
16 Dec'24 11:39 PM
16 Dec'24 11:27 PM
16 Dec'24 11:16 PM
21 Oct'24 04:01 PM
21 Oct'24 12:25 PM
Articles
Questions related to
Correct Answer: Sidgwick and Powell
Solution : The correct option is Sidgwick and Powell.
In 1940, Herbert Powell and Nevil Sidgwick proposed the notion that molecule shape and the number of valence electrons were correlated. They claimed that the number of valence electron pairs surrounding the core atom determines the shape of a molecule.
Correct Answer: 18
Solution : The correct answer is 18.
A precise circular path known as the shell is followed by electrons as they orbit the nucleus. The shells are either designated alphabetically with letters beginning with K (K, L, M, etc.) or according to the primary quantum numbers (n = 1, 2, 3, 4, etc.). Only a certain number of electrons can fit inside each shell. According to the formula, the nth shell has a theoretical maximum capacity of 2n² electrons. Based on this formula, the M shell can hold a maximum of 18 electrons.
Correct Answer: All the alkali metals have two valence electrons.
Solution : The incorrect statement is All the alkali metals have two valence electrons.
Group 1 of the periodic table comprises all alkali metals, which have a single valence electron. Electrons in the atom's outermost orbit are known as valence electrons. Group 1 of the periodic table contains the alkali metals hydrogen (H), lithium (Li), sodium (Na), potassium (K), caesium (Cs), rubidium (Rb), and francium (Fr).
Correct Answer: Erich Huckel
Solution : The correct option is Erich Huckel.
German scientist and physicist Erich Huckel put up a theory in 1931 to assist in identifying whether an aromatic property would be present in a planar ring molecule. According to his rule, an aromatic, cyclic, planar molecule possesses 4n+2 electrons. Hückel's Rule is the name given to this principle.
Correct Answer: Orbital
Solution : The correct option is Orbital.
The orbital motion describes electrons moving around the nucleus. Electrons orbit the nucleus at different energy levels called orbitals. These orbitals are specified areas of space in which an electron is likely to be discovered. Atomic electrons do not rotate around the nucleus; instead, they are constantly in motion and fluctuating in energy.

