Epimers - Definition, Glucose and Galactose, Examples, FAQs
In this article we will discuss about the epimers, epimers definition, then we will discuss the epimerization definition, then examples of epimers, their origin, how glucose and galactose are epimers, about epimeric pair, stating the names of epimers of glucose, what are enantiomers, what are enantiomers of glucose, what are stereoisomers, stereoisomers of glucose and each and every relevant information about the epimers.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What are Epimers?
- The Best Examples of Epimers are Glucose and Galactose
- Stereoisomers
- Enantiomers of Glucose
- Epimerization
What are Epimers?
Here we will discuss the meaning of word epimers and more precisely we will give here the epimers definition. So here we all know all types of sugar or sucrose comes under the category of carbohydrates that is consumed by us and here we are talking about the epimers which comes in observance when we have two sugars and they have almost same configuration but differ only at one position and that too for the placement of the hydroxide ions or the “OH” ions. So we can say that when the configuration of “OH” ions differ at one particular position in two different sugars then they are called epimers.
Also read -
The Best Examples of Epimers are Glucose and Galactose
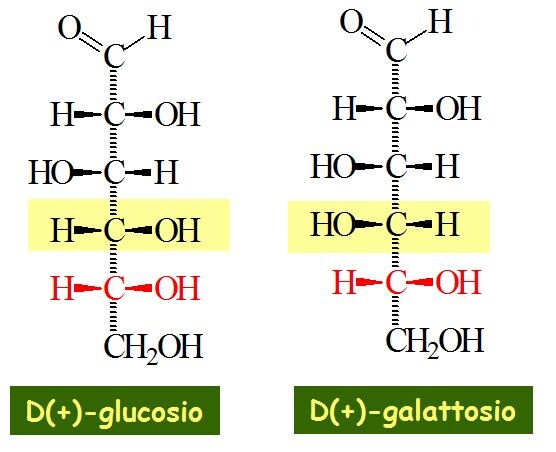
Now we will discuss in detail how glucose and galactose are epimers. When we see the structures of glucose and galactose we observe that it has almost the same configuration but the only difference which is observed is at the fourth carbon position otherwise the entire structure of the two is same. Now let us see what is the main difference at the fourth carbon. From the figure given below it is observed that the two structures are same but at the C4 the configuration of the OH ion is different in the two which further makes them different as glucose and galactose.
| Related topics link, |
Stereoisomers
We have a branch of chemistry known as stereochemistry. This branch of chemistry we call in common language is spatial isomerism. We are very much familiar with the structural isomers. As it is already known that isomers are those which have the same molecular formula but different possible structures that can be formed from that formula.
In the same way stereoisomers are those which have the same molecular formula of the compound but it has different possible spatial arrangements. Special arrangement is the three dimensional arrangement so formed in the same. Stereoisomers are further divided into diastereomers and enantiomers. Now we will discuss each in detail. Firstly we will discuss enantiomers.
So basically enantiomers are `arrangement of the isomers in such a way that they are optically different to one another. Here the isomers formed have mirror images of each other that means they give the same compound with the same formula and bonding among the different atoms but have opposite orientation as in mirror images.
It should be noted here that the mirror images are always non superimposable. These are called optical isomers. When we talk about the biological activities of any such compounds it should be noted that the enantiomers of a single compound have different activities in spite of the same physical properties. The difference in their mirror image leads to the change in the rotation of the compound.
Talking about the rotations they can be determined whether the compound rotates towards the right or it rotates towards the left. Depending upon their rotation they are being named differently. If the compound moves towards the right then it is called dextrorotatory and if it rotates towards left then it is called levorotatory.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
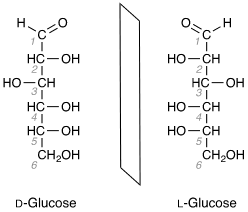
Enantiomers of Glucose
Discussing the enantiomers we have two types of enantiomers of glucose namely levorotatory and dextrorotatory. It is also symbolised as D- glucose and L- glucose. These two types of enantiomers off glucose are well known. It is important to know here that the glucose which we intake in our day to day life is dextrorotatory.
As it is already discussed that the compound moves towards the right then it is called dextrorotatory and if it rotates towards left then it is called levorotatory. Then we should also note here that the levorotatory are those which are now also synthetically synthesised that means they are synthesised in the laboratory. It is also not necessary that all the levo and dextro compounds are available in nature; sometimes their existence can not be surely known.
Talking about another type of stereoisomer that is diastereomers. This type of stereoisomers also have the same physical properties as the other isomers. These show the cis and trans isomerism. In cis and trans isomers the position of the functional group gets changed due to which the naming also gets changed. Now we will discuss some of the epimeric pairs known and those which are generally discussed in chemistry.
Basically epimeric pairs are those pairs which have different names due to differences in the structure but are only different by arrangement at one of the carbons in the entire chain. We have glucose and mannose which are both dextrorotatory in nature and differ from each other in the structure at the second carbon only.it is one of the most common examples used In chemistry. Lastly we will discuss the process of epimerization.
Epimerization
The process described here is of the epimers . A process in which different epimeric pairs form epimers in order to show the process of stereochemistry at one of the positions of the carbon atom , this process which it undergoes is called epimerization.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
This process of stereochemistry helps to bring stability to the compound and one such important use of stereochemistry in everyday life is in manufacturing of tetracycline which is an anticancer drug. The stability in the compound which is brought by this process is used to cure cancer.
The epimers which come into observance when we have two sugars and they have almost the same configuration but differ only at one position and that too for the placement of the hydroxide ions or the “OH” ions. So we can say that when the configuration of “OH” ions differ at one particular position in two different sugars then they are called epimers. The best examples of epimers are represented by glucose and galactose.
Discussing the enantiomers we have two types of enantiomers of glucose namely levorotatory and dextrorotatory. It is also symbolised as D- glucose and L- glucose the dextrorotatory is represented by + sign and levorotatory is represented by - sign.
Basically epimeric pairs are those pairs which have different names due to differences in the structure but are only different by arrangement at one of the carbons in the entire chain. We have glucose and mannose which are both dextrorotatory in nature and differ from each other in the structure at the second carbon only.it is one of the most common examples used in chemistry.
Stereoisomers are those which have the same molecular formula of the compound but it has different possible spatial arrangements. Special arrangement is the three dimensional arrangement so formed in the same.Stereoisomers are further divided into diastereomers and enantiomers.
Also Read
12 Dec'24 04:49 PM
04 Nov'24 05:31 PM
21 Oct'24 05:53 PM
09 Oct'24 06:12 PM
09 Oct'24 04:00 PM
30 Sep'24 10:57 AM
30 Sep'24 08:52 AM