Equilibrium
The concept of chemical equilibrium was developed over time through the contribution of various scientists including Henry Le Chatelier in 1884 and Cato Guldberg and Peter Waage in 1864. If the states of dynamic equilibrium is disturbed by changing the condition, the position of equilibrium shifts to counteract change.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Classification Of Equilibrium
- Chemical Equilibrium
- Types of reactions
- Some Solved Examples
- Summary
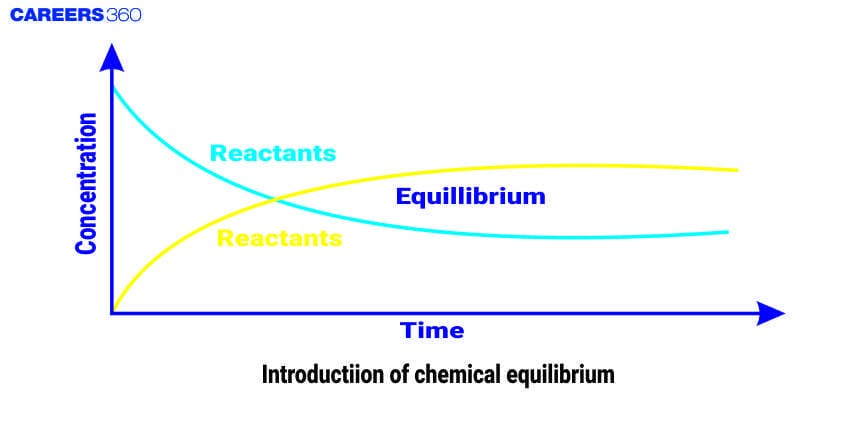
The word Equilibrium means a state of zero change or no change in the reaction. The chemical equilibrium is the state in a chemical reaction where the rate of forward reaction is equal to the rate of backward reaction which results in the constant concentration of the reactant and product with time.
At the equilibrium condition, the concentration of every single species remains unchanged, and the reaction continues to occur which means that the reaction is in a state of dynamic balance or stable condition. Equilibrium is of various types such as physical equilibrium, thermal equilibrium, mechanical equilibrium, chemical equilibrium, etc.
Classification Of Equilibrium
The word "equilibrium" in a physical sense is explained as the "No change of state of the body". When the two opposing processes (reaction) occur simultaneously at equal rates, the system is in a state of equilibrium. Equilibrium is classified as follows

When an equilibrium exists between the same chemical species, it is called physical equilibrium. For example:H2O(s)⇌H2O(l)
When an equilibrium exists between different chemical species, it is called chemical equilibrium.N2( g)+3H2( g)⇌2NH3( g)
If a chemical equilibrium has only one phase, it is called homogenous, and if more than one phase it is called heterogeneous.
Chemical Equilibrium
It is the state of a reversible reaction at which measurable properties like color, density, pressure concentration are nearly unchangeable.

Here V1 and V2 are the rates of forward and backward reactions respectively. i.e., equilibrium is the state in a reversible reaction at which the rate of forward and backward reactions or two opposing reactions are the same.
Types of reactions
Chemical reactions are of two types:
1. Irreversible Reaction: Such reactions occur in one direction only and are completed.
For example:(i) When unreactive products or solid products are formed.
NH4NO2→ΔN2+2H2O
(ii) All precipitate reactions are irreversible.
BaCl2+H2SO4→BaSO4+2HCl
(iii) Neutralisation reactions are also irreversible.
H2SO4+2KOH→K2SO4+2H2O
(iv) Redox reactions are also irreversible.
2FeCl3+SnCl2→SnCl4+2FeCl2
(v) Combustion reactions are also irreversible.
2Mg+O2→2MgO
2. Reversible Reactions: Such reactions occur in both directions i.e., forward and backward direction however never complete as the products can give back the reactants under the same or different conditions. For example:
N2+O2⇌2NO
- The vaporization of water in an open flask is an irreversible reaction while in a closed flask, it is reversible.
- The decomposition of CaCO3 in the open flask is an irreversible reaction while in the closed flask, it is reversible.
Recommended topic video on (Equilibrium)
Some Solved Examples
Example 1. Which of the following statements is/are true for the equilibrium between water and water vapor?
1)It has a constant vapor pressure
2) The rate of conversion of vapors to water and water to vapors becomes constant
3) The rate of conversion of vapors to water and water to vapors becomes the same
4) (correct)Both 1 and 3
Solution
As we have learned
Liquid Vapour equilibrium -When a liquid evaporates in a closed container molecules with relatively higher kinetic energy escape the liquid surface into the vapor phase and several liquid molecules from the vapor phase strike the liquid surface and are retained in the liquid phase. It gives rise to a constant vapor pressure because of equilibria.
e.g. H2O( liquid )⇌H2O( vapour )
For equilibrium between water and water vapors, the vapor pressure becomes constant and the rate of conversion of the two species becomes the same
Hence, the answer is the option (4).
Example 2. When the equilibrium is attained between reactant and product, which one of the following is correct for the same?
1) The rate of change of reactant to product and product to reactant becomes constant
2) The concentration of reactant and product becomes constant
3) The rate of conversion of reactant to product and product to reactant becomes the same
4) (correct)both (2) and (3)
Solution
When a reaction attains equilibrium, the rate of reaction of forward and backward reaction becomes the same and the concentration of reactants and products becomes constant.
Hence, the answer is the option (4).
Example 3. Equilibrium between ice and water (liquid ) is known as
1)Liquid - vapor equilibrium
2)Solid - vapor equilibrium
3) (correct)solid-liquid equilibrium
4)none of these
Solution
Physical equilibrium - If the two opposing processes involve only physical changes the equilibrium is called physical equilibrium.
E.g. solid-liquid equilibrium, liquid-vapor equilibrium, solid-vapor equilibrium
Equilibrium between ice and water is known as solid-liquid equilibrium
Hence, the answer is the option (3).
Example 4. Which of the following is/are true for the equilibrium between ice and water?
1)Both the opposing processes occur simultaneously
2)both processes occur at the same rate
3)The amount of ice and water remains constant
4) (correct)all of these
Solution
Solid-liquid equilibrium - ice ⇌ water
The system here is in dynamic equilibrium and we can infer the following
1. Both the opposing processes occur simultaneously
2. Both processes occur at the same rate so that the amount of ice and water remains constant
All of these statements are true for the equilibrium b/w ice and water
Hence, the answer is the option (4).
Example 5. During the process of transport and delivery of O2 from our lungs to muscle, there is an equilibrium b/w species
1)CO and the protein hemoglobin
2)NO and the protein hemoglobin
3) (correct)O2 and the protein hemoglobin
4)none
Solution
Equilibrium involving O2 molecules and the protein hemoglobin plays a crucial role in the transport and delivery of O2 from our lungs to our muscles. Similar equilibrium involving CO molecules and hemoglobin account for the toxicity of CO. In the given process, the equilibrium is between O2 and the protein hemoglobin
Hence, the answer is the option (3).
Summary
Chemical equilibrium occurs when the reaction reaches the state where the concentration of reactant and product remains constant with time, It only happens when the rate of both the backward and forward rates of reaction become equal to each other. Various other key points are included in this such as its dynamic nature, equilibrium constant, le chatelier principle, and other factors which affect the equilibrium conditions.
Also Read
07 Feb'25 12:04 AM
07 Feb'25 12:00 AM
06 Feb'25 11:50 PM
06 Feb'25 11:48 PM
06 Feb'25 11:40 PM
09 Dec'24 11:40 AM
21 Oct'24 12:32 PM
21 Oct'24 11:57 AM
19 Oct'24 03:14 PM
19 Oct'24 03:08 PM

