Examples of Gases - List of Gaseous with FAQs
Gas is a type of state of matter which has no shape, size, and volume compared to solid and liquid forms. Gas has a property that it takes the shape and volume of the container where it is present. For gaseous matter, the particles that are elements or compounds are arranged in such a way that the distance between two particles is very large. To find a particle in its gaseous form is difficult. Air is an example of a gas which contains different types of compounds. Air is present all around us and we cannot even think that it contains too many compounds of gases.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Properties of Gases
- Examples of gases
- Ideal Gas Versus Real Gas
- Gas Name
- Types of Gas
- Applications of Gases
Air is a mixture of nitrogen, carbon dioxide, oxygen, hydrogen, carbon dioxide, water vapour, and small amounts of other compounds like argon, neon, krypton, etc. The main component of air is nitrogen that is, 78 % of our air is filled with nitrogen gas. And then comes the oxygen about 20.9%. All the other gases in 0.17 %. At higher regions of air triatomic oxygen is found that is ozone.
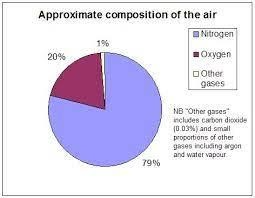
Figure showing the composition of air
Properties of Gases
Indefinite Shape and Volume - Gases take the shape and volume of their container.
Compressibility - Gases can be compressed due to the large spaces between particles.
Low Density - Gases have lower density compared to solids and liquids.
Diffusion and Effusion - Gases spread out and mix without external forces, and they can pass through small openings without collisions.
Pressure Exertion - Gas molecules exert pressure on the walls of their container due to their constant motion.
Also read -
Examples of gases
Some of the very common examples of compounds that are present in their gaseous form are described below.
Air
Argon
Nitrogen
Carbon dioxide
Helium
Oxygen
Ozone
Water vapour
For a molecule to be in its gaseous form requires certain conditions. But many elements can be accessed in their gaseous form even at room temperature and at ordinary pressure. They are hydrogen, oxygen, nitrogen, chlorine, and fluorine. And they are present in their diatomic form. That is H2, O2, N2, Cl2, and F2. Oxygen tends to form gas even in its triatomic former and it is the very important compound ozone that is O3 protects us from severe ultraviolet radiation.
The noble gases as the name suggests that noble gases have the property of existing in gaseous form even at room temperature and it is present in its monoatomic form. That is He, Ne, Ar, Kr, Rn, etc. are all gases and given the name pure gases. Water is a compound that can exist in the vapour form that is the gaseous former only at a particular temperature and pressure. Water is a compound that is in liquid form at room temperature and ordinary pressure. The gaseous form of water at a particular temperature and pressure is called water vapour.
Ideal Gas Versus Real Gas
A gas that behaves based on the Kinetic molecular theory and according to the law of ideal gas is ideal. This means that the particles present in an ideal gas are not attracted to each other have no volume and their interaction is perfectly elastic. This fact is the only theoretical basis not any gas in the world is not ideal. But under certain conditions that is at ordinary temperatures and pressures, their behavior is sometimes close to the ideal gas behavior that is verified by the use of ideal gas law.
The behaviour of real gases deviates from ideality at low temperatures and high-pressure conditions. The reason behind this is that under high pressure the molecules of gases come close to each other and the behavior of gas is lost. Also at high pressure, a gas molecule can be compressed into its liquid form itself. So then what is the case of low-temperature, the particles present in the gases need the energy to vibrate and to interact. If the temperature is low the kinetic energy of the particle will be low and the nature of perfect elastic collision has been lost.
Related Topics, |
Gas Name
Some of the gas name and their formula is given below.
Name | Formula |
Carbon dioxide | CO2 |
Oxygen | O2 |
Nitrogen | N2 |
Helium | He |
Ozone | O3 |
Fluorine | F2 |
Types of Gas
Some of the elements can exist in their gaseous form at the standard temperature for ordinary temperature and pressure but certain other elements cannot exist in these circumstances but can exist by applying certain conditions on it. Some of the types of gases based on this are described below.
Elemental Gases
The type of gases that can exist in their gaseous form even at the standard temperature or ordinary temperature and pressure are elemental gases. If the pressure or temperature is raised or lowered it can exist in a different form that is liquid form and its solid form. Examples of these elemental gases are hydrogen, oxygen, helium, fluorine, chlorine, etc.
Pure Gases
The gas that is made up of their atom that is present in a monoatomic former are called up you are gases. Examples of these included all the noble gases such as neon, helium, argon, krypton, etc. The picture of gases is shown below.

Mixed Gases
The gases that contain more than one element or more than one atom are mixed gases that are it is a mixture of different gases. Carbon dioxide is one of the very important examples as it is a mixture of carbon and oxygen. Acetylene C2H2, butane C4H10, ethane C2H6, methane CH3, Sulphur hexafluoride SF6, etc. are gas mixture examples.
Toxic Gases
The gases that are toxic which means can cause harm to people when they breathe. Ozone is a toxic gas. Carbon monoxide, hydrogen bromide, hydrogen chloride, ozone, nitrogen dioxide, etc. are some of the poison gas names.
Applications of Gases
Gases are widely used in various industries and daily life:
Oxygen (O₂) - Essential for respiration and medical applications.
Nitrogen (N₂) - Used in food preservation and industrial processes.
Helium (He) - Used in balloons, MRI machines, and deep-sea diving.
Hydrogen (H₂) - Used in fuel cells and industrial synthesis.
Carbon Dioxide (CO₂) - Used in carbonated beverages, fire extinguishers, and plant growth
Frequently Asked Questions (FAQs)
An ideal gas follows the ideal gas law (PV=nRT) under all conditions, assuming no intermolecular forces and negligible molecular volume. In contrast, real gases deviate from this behaviour at low temperatures and high pressures due to intermolecular attractions and the finite volume of molecules.
Gases have no fixed shape or volume because their molecules are in constant random motion and spread out to occupy any available space. This behaviour is due to the weak intermolecular forces and high kinetic energy of gas particles.
There are mainly four types of gas they are; elemental, mixed, pure, and toxic gas.
Carbon monoxide is a colourless, odourless gas that binds to haemoglobin in the blood more effectively than oxygen. This reduces oxygen transport in the body, leading to poisoning, which can be fatal in high concentrations.
According to Charles’s Law, gas volume increases with temperature when pressure is constant. This is because higher temperatures increase the kinetic energy of gas molecules, causing them to move faster and spread out.
Also Read
11 Mar'25 12:08 PM
19 Feb'25 11:49 AM
19 Feb'25 11:23 AM
19 Feb'25 11:14 AM
19 Feb'25 10:31 AM
18 Feb'25 12:15 PM
18 Feb'25 11:59 AM
09 Feb'25 10:16 PM
08 Feb'25 05:31 PM
08 Feb'25 05:28 PM