Formal Charge And Its Properties
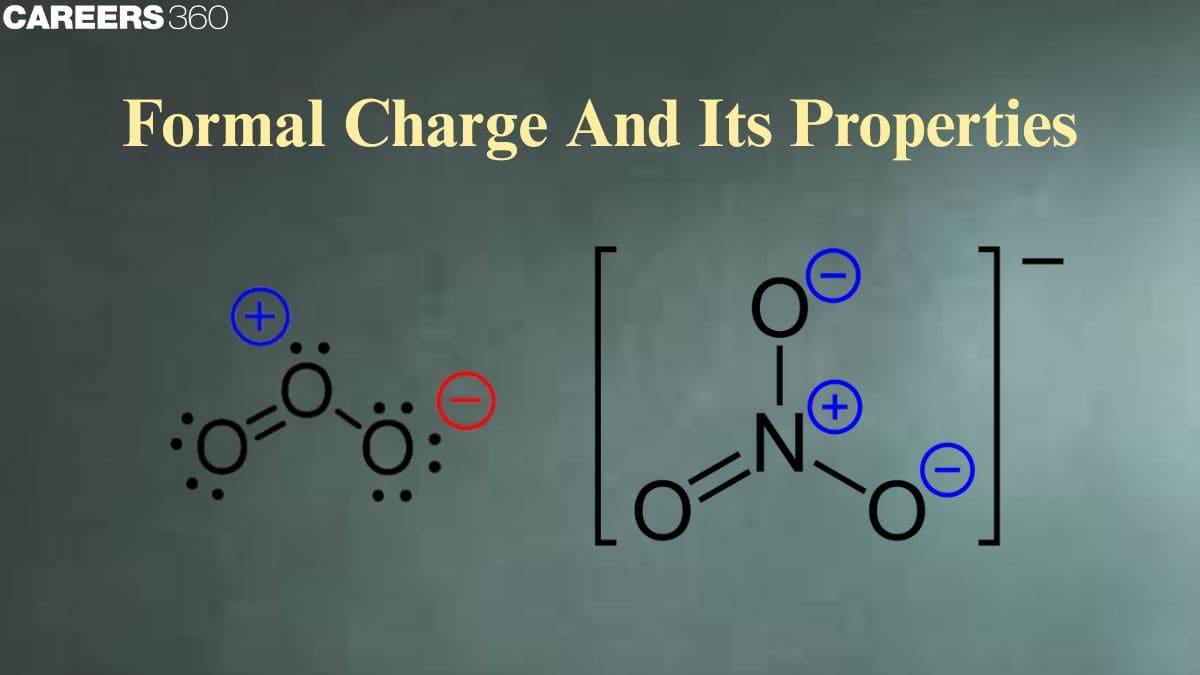
Formal charge is a concept in chemistry central to the understanding of molecular structure and stability. It's explained as the hypothetical charge assigned to an atom in a molecule on assuming that electrons in chemical bonds are shared equally between the bonded atoms, regardless of their difference in electronegativity. It is an overly simplified concept for the prediction of the most stable Lewis structure of a molecule that would help the chemist trace how an arrangement of atoms would minimize both the overall charge and energy of the molecule. The formal charge is described as the sum of all an atom's valence electrons in a molecule and its nonbonding electrons with the bonding electrons. It is computed via a definite formula.
This Story also Contains
- Understanding Formal Charge The formal charge is calculated using the following formula
- Formal Charge
- Types and Importance of Formal Charge
- Applications of Formal Charge in Real Life
- Some Solved Examples
- Summary
Also Check:
Understanding Formal Charge
The formal charge is calculated using the following formula
$\mathrm{FC}=\mathrm{V}-\mathrm{N}-2 \mathrm{~B}$
where:
- V refers to the number of valence electrons of the neutral atom in its ground state.
- N is the number of nonbonding valence electrons (lone pairs) on the atom in the molecule.
- B is the total number of electrons shared in bonds with other atoms.
This equation comes in handy when a chemist is trying to calculate the formal charge on every atom in a molecule, simply to compare different Lewis structures. An ideal Lewis structure of the molecule would be the one in which formal charges on atoms are as close to zero as possible, describing a very stable configuration—for instance, CO₂, where every atom in a most preferred Lewis structure has a formal charge of zero, hence manifesting itself as a very stable arrangement.
Formal charge is, therefore, a fiction, but very useful in elucidating electron distribution in a molecule. In that regard, formal charges should not be thought of as actual charges on atoms, but rather, they are there just to give a guide to the understanding of the stability and reactivity of molecules.
Formal Charge
The molecule is neutral and its constituent atom do not carry any charge, similarly in case of polyatomic ions, the net charge is possessed by the ion as awhole and not by a particular atom. Yet for some purposes, it is very useful to assign formal charges to the individual atoms constituting molecules or even polyatomic ions.
The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. In other words, formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons and then subtract the number of bonds connected to that atom in the Lewis structure.
Thus, we calculate the formal charge as follows:
Formal charge = valence shell electrons − lone pair electrons − 1/2 bonding electrons
Also Read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Using Formal Charge to Predict Molecular Structure
The arrangement of atoms in a molecule or ion is called its molecular structure. In many cases, following the steps for writing Lewis structures may lead to more than one possible molecular structure—different multiple bonds and lone-pair electron placements or different arrangements of atoms, for instance. A few guidelines involving formal charge can help decide which of the possible structures is most likely for a particular molecule or ion:
-
A molecular structure in which all formal charges are zero is preferable to one in which some formal charges are not zero.
-
If the Lewis structure has non-zero formal charges, the arrangement with the smallest non-zero formal charges is preferable.
-
Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign.
-
When we must choose among several Lewis structures with similar distributions of formal charges, the structure with the negative formal charges on the more electronegative atoms is preferable.
Let us consider some possible structures for carbon dioxide, CO2, and thiocyanate. We know that the less electronegative atom typically occupies the central position, but formal charges allow us to understand why this occurs. We can draw three possibilities for the structure: carbon in the center and double bonds, carbon in the center with a single and triple bond, and oxygen in the center with double bonds:

Comparing the three formal charges, we can identify the structure on the left as preferable because it has only formal charges of zero (Guideline 1).
In the case of thiocyanate ion, an ion formed from a carbon atom, a nitrogen atom, and a sulfur atom, three different molecular structures: $\mathrm{CNS}^{-}, \mathrm{NCS}^{-}$, or $\mathrm{CSN}^{-}$ are possible as shown below. The formal charges present in each of these molecular structures can help us pick the most likely arrangement of atoms. Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown below:

Note that the sum of the formal charges in each case is equal to the charge of the ion (–1). However, the first arrangement of atoms is preferred because it has the lowest number of atoms with nonzero formal charges. Also, it places the least electronegative atom in the center and the negative charge on the more electronegative element.
Also Read:
Types and Importance of Formal Charge
Formal charge comes to the forefront in several situations, mostly in the knowledge of the preferred structure of a molecule. Given below are some of the main aspects of formal charge:
- Multiple Lewis Structures: For those cases when more than one Lewis structure can be drawn, one with the least formal charge usually is preferred.
- Example: In ozone (O3) resonance structures can be drawn and formal charges help in understanding the stabilization of resonance hybrid.
- Reactivity Predictions: Formal charges do help in predicting the reactivity of molecules.
- Negative Formal Charge: Atoms bearing negative formal charges are more likely to behave as nucleophiles.
- Positive Formal Charge: An atom with a positive charge exhibits electrophile-like behavior.
- Geometry and Polarity of Molecules: Formal charge enables one to predict molecular geometry and polarity, which are features that impact the physical and chemical properties of the molecule.
Applications of Formal Charge in Real Life
- The concept of formal charge has far-reaching applications in the academic as well as practical fields:
- Reaction Mechanisms: Formal charges play a huge role in predicting the stability and reactivity of intermediates involved, like carbanions or carbocations, in a reaction.
- Example: The distribution of formal charge across the molecule influences the stability of a carbocation.
- Enzyme-Substrate Interactions: Formal charge determines how the binding of substrates at active sites takes place and thus influences biological processes.
- Example: The side chains of amino acids could be modulating protein folding and function through their charges.
- Case Study: Designing inhibitors of enzymes at a time requires formal charge consideration that needs attending to for their efficacy enhancement.
Related Topics:
Recommended topic video on ( Formal Charge)
Some Solved Examples
Example 1
Question: In the $({PO}_4^{3-} )$ ion, what is the formal charge on the oxygen atom of the P-O bond?
Solution:
To determine the formal charge on the oxygen atom in the $({PO}_4^{3-} ) $ion, we use the formula:
$[{Formal charge} = text{valence electrons} - text{lone pair electrons} \frac{text{bonding electrons}}{2}]$
Valence electrons for oxygen = 6
Lone pair electrons on oxygen = 6 (since there are three lone pairs on each oxygen)
Bonding electrons P-O = 2 (since one bond is present)
Thus, the formal charge on each oxygen atom in a single P-O bond is:
$[6 - 6 - \frac{2}{2} = -1]$
Therefore, the correct answer is : -1.
Example 2
Question: In the $({O}_3 )$ molecule, what is the formal charge on the central oxygen atom?
Solution:
To find the formal charge on the central oxygen atom in $( \text{O}_3 )$, we use the formula:
Valence electrons for oxygen = 6
Lone pair electrons on central oxygen = 4
Bonding electrons(two bonds with adjacent oxygens) = 4
The formal charge is calculated as follows:
$[6 - 4 - \frac{4}{2} = +1]$
Hence, the correct answer is : +1.
Example 3
Question: What is the formal charge on the nitrogen atom in $({NO}_2^- )$?
Solution:
For the nitrogen atom in $({NO}_2^- )$, the formal charge can be calculated as:
Valence electrons for nitrogen = 5
Lone pair electrons on nitrogen = 2
Bonding electrons (two bonds with oxygen atoms) = 6
Thus, the formal charge is:
$[5 - 2 - \frac{6}{2} = 0]$
The correct answer is: 0.
Example 4
Question: In $({SO}_4^{2-} )$, what is the formal charge on the central sulfur atom?
Solution:
The formal charge on the central sulfur atom in $({SO}_4^{2-} )$ is determined by:
Valence electrons for sulfur = 6
Lone pair electrons on sulfur = 0
Bonding electrons(four bonds with oxygen atoms) = 12
The formal charge is:
$[6 - 0 - \frac{12}{2} = 0]$
Therefore, the correct answer is 0.
Example 5
Question: In $({PO}_4^{3-} )$, what are the formal charges on each O atom and the P-O bond order, respectively?
Solution:
1. Formal charge on each O atom:
The total charge on $({PO}_4^{3-})$ is -3, distributed over 4 oxygen atoms.
$[{Formal charge per O atom} = \frac{-3}{4} = -0.75]$
2. P-O bond order:
The P-O bond order is calculated based on the resonance structures:
$ [{Bond order} = \frac{{Total number of bonds}{Number of resonance structures}}=\frac{5}{4} = 1.25]$
Thus, the correct answer is : -0.75, 1.25.
Example 5:
The formal charge on central oxygen atom in ozone is:
[JEE Main 2024]
1) 0
2) +2
3) +1
4) -1
Solution:
The figure is given below:

Formal charge $=$ valence - non bonding - bonding electrons
For1: $\quad 6-4-\frac{4}{2}=0$
For2: $\quad 6-2-\frac{6}{2}=6-2-3=1$
For3: $\quad 6-6-\frac{2}{3}=-1$
Hence, the answer is the option (3).
Practice More Questions From the Link Given Below:
Summary
Formal charge, therefore, is a very important conceptual theory in chemistry that gives insight into electron distribution within a molecule. Formal charge allows a chemist to work out the most stable Lewis structure of a molecule—one with the least formal charge. Formal charge helps in determining the most stable lewis / resonance structure. Among possible ways of arranging multiple bonds, lone pairs, etc., the structure(s) that minimize formal charges (especially large ones) tend to be more stable. Also, negative formal charges are preferably placed on more electronegative atoms. In transition metal chemistry, formal charges might be ambiguous; electron counts, oxidation states, coordination geometry, ligand field effects complicate simple picture. Formal charges may be omitted in many inorganic texts unless needed.
Also Check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Formal charge is a theoretical charge assigned to an atom in a molecule assuming that electrons in bonds are shared equally. It helps in drawing Lewis structures and understanding charge distribution.
Use the formula:
Formal charge = (Valence electrons of free atom) − (Non‑bonding electrons on that atom) − (½ × shared electrons in bonds)
Alternatively, one can think of it as valence electrons minus (number of lone‐pair electrons + number of bonds).
No. Formal charge is a bookkeeping model; actual charge distribution depends on many factors like electronegativity differences, molecular orbitals, resonance, and environment. Formal charge gives a simplified picture, not the full one.
When there are several resonance forms, the one with better (lower) formal charges often contributes more.
When deciding if double/triple bonds or expanded octets are needed to attain lower formal charges.
In certain high‐oxidation state compounds, where balancing formal charge helps in writing correct bonding arrangements.
In electron‐counting of metal centers, to verify ligand charges and overall complex charge.
To decide among possible structures of ligands (which atoms are bonded, multiple vs single bonds etc.).
To predict which ligand or atom in the complex may be more reactive or donor/acceptor based on its formal charge.
Yes. Even in stable compounds or ions, individual atoms can bear positive or negative formal charges. The total of all formal charges must equal the overall charge of the species.
