Fractional Distillation - Examples, Distillation, Diagram, FAQs
What is Fractional Distillation?
Here as the name suggests the fractional distillation, we should know the meaning of the word distillation. So, basically distillation is the process used in chemistry to separate the mixture into its constituent liquids using either the process of boiling as we know that that different liquids have different boiling points or by the process of condensation with the same criteria that the liquids cool at the different temperatures.
Here we are talking about the fractional distillation as the name suggests that that the process of distillation takes place in fractions and fractions are the parts or the layers. From this above discussion fractional distillation definition will be given as the separation of the mixture of the fluids into different layers when they are boiled as the different components boil at different temperatures.
Also read -
Fractional Distillation Examples
As we know that there are a lot of impurities in drinking water which cannot be easily seen so they are removed by this method of fractional distillation.
Like we are here talking about the water in the same way air is also full of impurities and some of those impurities can be removed by the fractional distillation.
It is further used in refining of oil. This we will discuss in detail in this article only.
It is also in manufacturing of alcohol.
One of the common use is manufacturing of the perfumes and this is a skin product so each and every quantity used should be very precise therefore, this method is used.
As we have discussed what actually the fractional distillation is so here we need to discuss the diagram of same as well but before that we need to discuss the type of liquids or the mixtures in which such a process takes place. We should always remember that fractional distillation is suitable for miscible liquids. In chemistry there are two types of liquids namely miscible and immiscible liquids. Miscible liquids are those which are well mixed with each other and immiscible liquids are those which are not well mixed and there is difference always visible.
Fractional Distillation Diagram
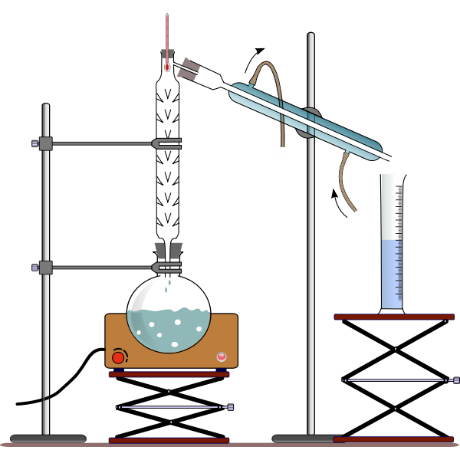
For explaining any diagram we should know the apparatus required for this which will include burner, round bottom flask, a long column called fractionating column, thermometer for measuring temperature, a flask for collection of filtered liquid and condenser.
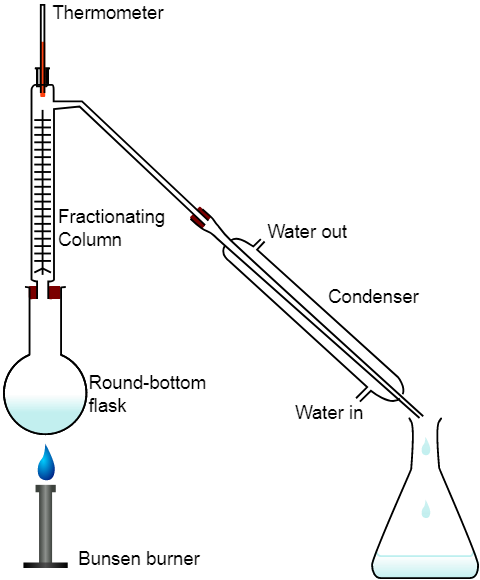
Now we will discuss how it works:
In this diagram as we can see that there is liquid filled in the round bottom flask and then burner is set on and it is seen that as the liquid gets heated up it gets converted into vapours and vapours being lighter in weight rises above and goes into the fractionating column where there are glass beds present which allows vapours to condense and liquid in the mixture, being lighter one of them moves through the glass tube and condenser and gets collected into flask and this way the liquids of the mixture gets separated. Now as we have discussed the diagram and it’s time to discuss on some of the uses of fractional distillation.
Related Topics, |
Fractional Distillation of Petroleum Class 8
Petroleum is extracted from the crude oil which is the raw form of the petroleum and this crude oil is refined into different products through fractional distillation. When the crude oil is taken in the fractional distillation apparatus, it has various constituents mixed which have different boiling points and this is the main principle of fractional distillation. When the crude oil gets refined it gets converted into petrol, kerosene, paraffin wax, petroleum gas. Here as we are discussing about the products so obtained from petroleum but before that we need to discuss the process of separating useful fractions from petroleum.
As we all know that the petroleum is extracted from the sea beds. From the sea beds the crude oil which is raw form of petroleum is extracted using drills and pipes and then this crude oil is heated to about 600 degree celcius which is really a high temperature , then as it is known then it gets converted into vapours in the fractional distillation apparatus and further gets condensed into fractionating column as the temperature in the column is about 20 degree celsius and then the vapours or the constituents of the crude oil with high boiling point remains at the bottom and those with the low boiling point remain at the top and then moves through the glass tube followed by the condenser and then gets collected in the collection flask.
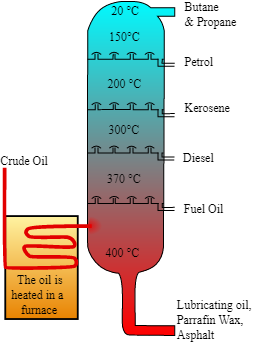
The different products so obtained are petrol, kerosene, paraffin wax, petroleum gas each having its own importance in day to day life.
Petrol: It is used to run automobiles basically as a fuel.
Petroleum gas: It is used as fuel in the kitchens to cook food.
Kerosene: It is also used to cook food and also in laboratories.
Paraffin wax: It is used on skin to keep it moisturised like Vaseline jelly.
Also, students can refer,
- NCERT Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of
elements - NCERT Exemplar Class 12 Chemistry solutions Chapter 6 General Principles and Processes of isolation of
elements - NCERT notes Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of elements
Distillation
Distillation of miscible liquids involve the process of boiling the miscible liquids to certain temperature. Distillation is based on the principle according to class 9 is that of selective boiling and condensation.
The method of distillation apparatus involves: a round bottom flask, a burner as a heat source, a thermometer, a condenser with water in and water out mouth and then flask for the product collection.
Distillation Diagram
The diagram of distillation is shown below:
The distillation method involves the boiling of the miscible liquids to their boiling temperature which allows the vapours to rise and then pass through the condenser to get collected in the collection flask.
The only difference in the apparatus of the distillation and fractional distillation is the presence of fractionating column.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: