Froth Floatation Process
Consider the separation of the worthless rock as a way to save valuable minerals from churning out raw materials for the modern world. All this is a complicated method to go about; it is called froth flotation, the extraction of minerals such as copper, lead, and zinc from ores. But the origin of many modern necessities, from smartphones to buildings, dates back to this amazing process. It reveals not only a vein of knowledge and ingenuity in chemistry and engineering but also an excellent example of how scientific ingenuity enhances industrial productivity.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Definitions of the Froth Flotation Process
- Chemical and Mechanical Means of Froth Flotation
- Collectors and Depressants
- Applications and Significance of Froth Flotation Process
- Some Solved Examples
- Summary
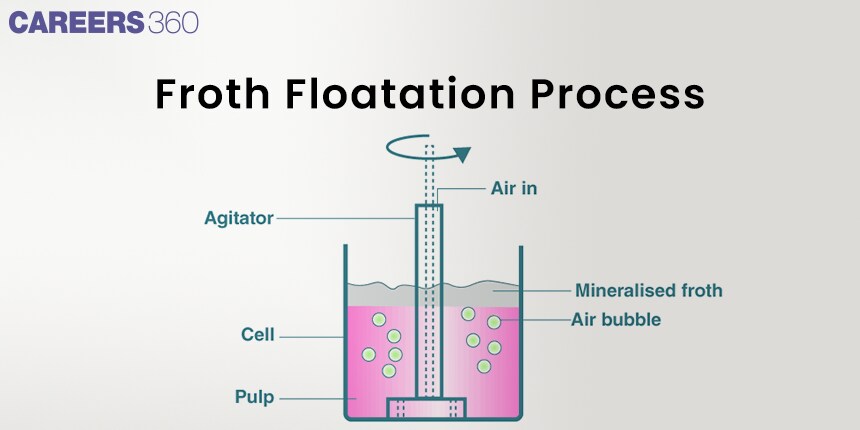
Definitions of the Froth Flotation Process
Any of several processes for separating ores. The surface properties of minerals are changed by froth flotation because some reagents are dissolved on the surface, or a film of air bubbles is created on the surface.
In the Froth Floatation process, you will come across different terminologies. Let's discuss them.
- Frothers - Frother is an agent that is active in froth flotation through its ability to change the surface tension of a liquid and generate a stable froth that rises to the top of the tank. Example - pine oil, Eucalyptus oil, etc.
- Froth stabilizers - Froth stabilizers are those substances that help stabilize the froth formed during the froth floatation process. Examples - cresols, aniline, etc.
- Collectors - A collector is a chemical that selectively binds to the surface of target minerals and imparts hydrophobicity to those mineral particles, a necessary condition for air bubble attachment. Example - pine oils, fatty acids, xanthates, etc.
- Depressants - These reagents depress the flotation property and help in the separation of different sulphide ores present in a mixture. We can say ‘Depressants’ are used for adjusting the proportion of oil to water to separate two sulphide ores. Example: ore containing ZnS and PbS, the depressant used is NaCN.

Reagents enable the stabilization of froth, the layer at the interface of separation from which the valuable mineral can be collected.
Froth Stabilizers
They are the chemicals used to increase the stability of the froth to make sure that the bubbles do not rupture too fast in order to ensure the effective separation of the valuable mineral. They are also widely used in the water treatment process for the same reasons, as they act as flocculant agents in the sense of gathering fine mineral particles together for efficient separation.
Collectors
These are in the form of hydrophobic chemicals that become ally attached to the surface of the preferential mineral particles in a solution where one absorbs selectively unto the surface of the preferential mineral particles.
Depressants
Chemicals that inhibit the formation of froth with certain kinds of particles by making them hydrophilic (water-attracting) and hence keeping it suspended in the pulp.
Chemical and Mechanical Means of Froth Flotation
Further and Froth Stabilizers
Addition of such frothers and froth stabilizers is important for the development of a froth layer required to carry the valuable minerals. Frothers are substances added into a slurry to form bubbles and include pine oil, alcohol, and other chemicals. Froth stabilizers are used to prevent these bubbles from bursting and induce a state in which a froth is created where minerals that have accumulated can be salvaged from this froth layer.
Collectors and Depressants
In this relation, the work of the collectors can be said to be a basis for the determination of what kind of minerals are going to be available in the slurry while, on the other hand, the depressants define what is going to be absent. For example, xanthates stick to the surface of the desired minerals making them hydrophobic; hence attracted to the bubble of the air with high affinity to float to the surface. For example, sodium cyanide is a depressor, in the fact that it is reversed: it makes the unwanted mineral hydrophilic and assures in the pulp, but gets it uncollected.
Copper Flotation
This is the process of recovering hydrophobic copper minerals from the gangue by froth flotation in the mining of the metal. It broadens the hydrophobicity of the copper mineral by adding potassium ethyl xanthate to further follow the rise of froth.
Lead-Zinc Flotation
In the mining process of extraction of lead and zinc, a beneficiation process is needed. This process isolates the metals from the ore. Collectors such as Di thiophosphates are allowed to act in the extraction of zinc, whereas depressants such as sodium cyanide make sure the other minerals are not wanted as interferers.
Applications and Significance of Froth Flotation Process
Industrial Applications
Froth flotation is highly relevant to the mining industry since it is the process that can be used to extract and purify minerals critical for other large-market applications. For example, copper from flotation can be used in electrical wiring, plumbing; lead and zinc are important components of batteries and in the process of galvanization. This technique has even been used for recycling purposes to recover these important materials from products that have been worn out.
Academic Applications
Flotation studies are categorized in mineral processing, chemical engineering, and metallurgy in academia. The process of froth flotation is often taken as a case study for the application of chemical principles to engineering problems. This kind of student or researcher work has the optimum performance of the process that needs to be attained with maximum effectiveness, minimum costs, and minimum environmental pollution in performing the process. The latest progress and development in flotation chemistry and technology toward more performance with enhanced sustainability of the method:
Future Trends
On the note of advances in froth flotation chemistry, researchers need to develop greener frothers and collectors afterward, which is a requirement of the industry to ensure a safe environment with the least possible detrimental effect and look for different ways to minimize dependence on the deleterious chemicals. Besides, progress in automation and control in flotation operations allows assemblies to be more accurate and efficient, providing an assurance of better management of resources with lesser environmental footprints.
Recommended topic video on(FrothFloatationProcess)
Some Solved Examples
Example 1
Question:
The role of pine oil in the froth floatation process is to enhance the non-wettability of:
1) Mineral particles in froth
2) Mineral particles in water
3) Gangue particles in froth
4) Gangue particles in water
Solution:
Collectors, such as pine oil, are used as frothing agents to increase the non-wettability of ore particles with water. Thus, pine oil enhances the non-wettability of mineral particles in water.
Hence, the answer is option (2).
Example 2
Question:
The correct statement is:
1) Aniline is a froth stabilizer
2) Zincite is a carbonate ore
3) Sodium Cyanide cannot be used in the metallurgy of silver
4) The zone refining process cannot be used for the refining of Germanium
Solution:
Aniline is a froth stabilizer. It is added to stabilize the froth.
Hence, the answer is option (1).
Example 3
Question:
Which of the following works as a depressant in the separation of ZnS and PbS?
1) AgCl
2) BaCl2
3) NaCN
4) CuSO4
Solution:
NaCN is an example of a depressant that is used to suppress zinc sulphide (ZnS) from entering the froth phase.
ZnS+4NaCN→Na2[ZnCN)4]+Na2S]
Hence, the answer is option (3).
Summary
Froth flotation is a very critical process in the mining industry. It deals with the separation of valuable minerals from their respective ores. As shown in the present article, from its beginning in terms of mineral separation, most parts of the process are covered with frothers, froth stabilizers, collectors, and depressants. We have discussed here the aspects and types of the process, its real-life examples, and considered its applicability in relation to diversification in various branches of industry and science. The applicability of froth flotation can be understood by a combination of its chemistry, engineering, and sustainability.
Also Read
06 Feb'25 11:59 PM
06 Feb'25 11:54 PM
09 Dec'24 11:00 AM
21 Oct'24 04:24 PM
07 Oct'24 02:35 PM
07 Oct'24 02:24 PM
07 Oct'24 02:17 PM
07 Oct'24 12:55 PM
04 Oct'24 07:17 PM