Functional Groups - Definition, Organic Compounds, Classes, FAQs
The property of an organic compound will depend on the substituent or moiety present in it. Functional groups are a group of an atom that is even attached to an organic or bond that changes the chemical and physical property of an entire molecule. The different functional groups in an organic molecule the change in chemical properties drastic and also by different the molecule to which the functional group is attached the property will also change.
This Story also Contains
- Functional Groups List
- Functional Group Class 10
- Functional Groups Table
- Some Solved Examples
- Conclusion
Functional Groups List
Functional groups can be charged for uncharged. Functional groups that contain any of the charges that are positive or negative are common one such example is COO- that is carboxylate. And uncharged molecules do not contain a charge on them example OH functional group. Functional groups are ligands when it gets bonded to a coordination complex that is forming a coordination bond with metal atoms.
The presence of certain functional groups on a molecule also affects its solubility for example sugar is a molecule that contains OH group attached to it and is easily dissolvable in water which means that when a highly electronegative group here oxygen is get attached to a less electronegative atom or molecule hair hydrogen the polarity increases and thereby the solubility in water increases. Some of the functional groups are described below.
Hydrocarbons
Those functional groups that are hydrocarbons when they contain hydrogen and carbon atom. Alkanes, alkenes, alkynes, and benzene are some of the functional groups in hydrocarbons these are generally represented by the simple R. It also has the name hydrocarbyl group. The reactivity of these types of compounds is a dependent bond present in between the carbon atom that is a double or triple bond. And also reactivity depends on whether it is cyclic or acyclic. It is also present in the charged ions and uncharged form charged hydrocarbons are called carbocations with a positive charge and carbanions with a negative charge.
Alkyl Halides
Alkyl halides are functional groups that contain carbon and halogen bond. For the notation of this species, the name ‘halo’ is used. An example of this is CH3Cl with the name methyl chloride. Depending on the type of halogen present on them the stability and the strength of these bonds change. For example, the carbon-fluorine bond is highly strong and highly stable compared to the carbon-iodine bond. Except for alkyl fluorides, all other haloalkanes undergo elimination and nucleophilic substitution reactions.
Oxygen-Containing Functional Groups
The functional group which contains an oxygen atom is the oxygen-containing functional group. It contains a carbon-oxygen bond. The bond present on them may be a double bond that is SP2 hybridization and a single bond SP3 hybridized. The reactivity is also very is with the presence of a bond between them. There are various types of functional groups containing carbon-oxygen bonds so the naming also varies with the type of atom to which it is attached. Amide is an example where it contains nitrogen also.
Nitrogen-Containing Functional Group
The functional groups that contain nitrogen atoms are nitrogen-containing functional groups. These compounds are given the common name amine represented by NH2. These are means may be primary, secondary, and tertiary as well. Depending on the number of carbon atoms present the naming also varies. If it contains only a single carbon atom can be named methylamine and if it contains two carbon atoms it is named dimethylamine and if it is 3 it is trimethylamine.
Functional Group Class 10
The groups of atoms or substituent or moiety get attached to an organic compound and thereby changing the whole molecule’s chemical and physical properties. And the new compound formed has property entirely different from the parent molecule. For example, benzene and phenol where an OH group is attached to the molecule. Properties of phenol and benzenes are extremely different.
Figure showing the structure of benzene
Figure showing the structure of phenol
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Priority Order of Functional Groups in IUPAC
For representing the position where the functional group is attached a priority order is necessary when a compound contains more than one functional group. From the IUPAC convention, the one with greater priority is carboxylic acid derivatives which include amides also. Of which amides have the highest priority then only carbonyls later alcohols then amines, alkenes, alkynes, and at last alkanes. If the compound contains a branch the longest carbon chain that is the one with higher carbon atoms is selected and is the parent compound. Anhydrides, esters, acid halides, acid halides have more priority than amides as well. The suffix for functional groups is shown in the functional group table.
The priority order for the functional group can be represented as;
Anhydrides>Esters>Acid halides>Amides>Nitriles>Alcohol>Amines>Alkenes>Alkynes>Alkanes
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
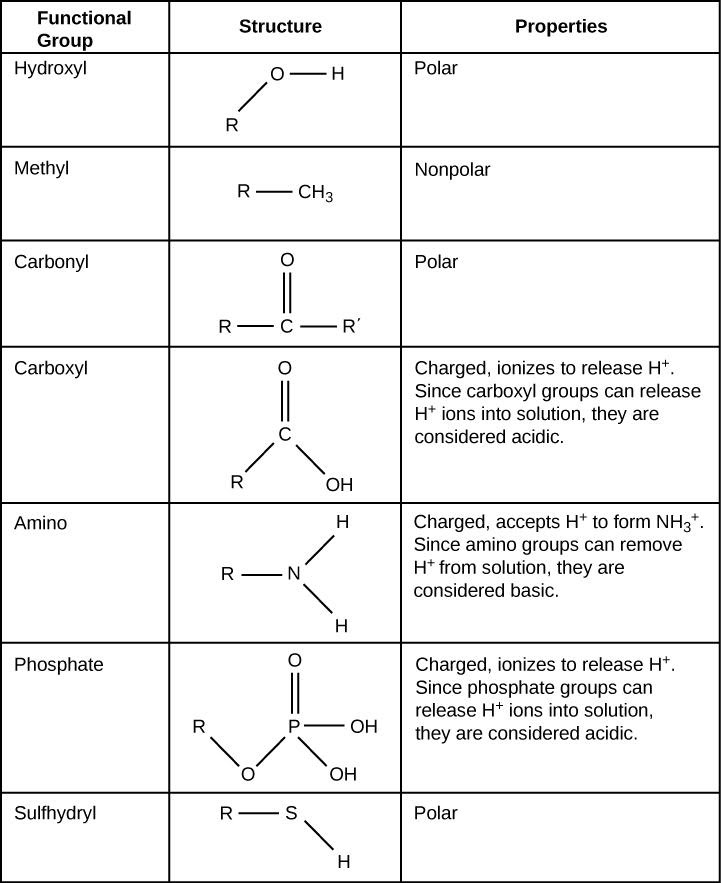
Functional Groups Table
The following figure will show the names and structure of some of the commonly used functional groups.
Recommended topic video on (Functional Group)
Some Solved Examples
Q 1. Compounds consist of particular atoms or groups of atoms on which all chemical properties depend known as -
(1) Prominent group
(2) Atom Group
(3) function group
(4) functional group
Solution:
As we learned
Functional group -
An atom, or group of atoms on which almost all chemical properties depend, is called a functional group.
$-\mathrm{COOH},-\mathrm{SO}_3 \mathrm{H},-\mathrm{COOR},-\mathrm{R}-\mathrm{COX}$
Hence, the answer is the option (4).
Q.2 Which of the following is not correctly matched to the name of the functional group?
1)ROH: Alcohol
2)RCOOH: Carboxylic Acid
3)$\mathrm{RCONH}_2$ : Amide
4) (correct)RCHO: Ketone
Solution
RCHO represents an Aldehyde and not a Ketone.
Hence, the answer is the option(4).
Conclusion
In organic chemistry, a functional group is a specific group of atoms or bonds within a compound that is responsible for the characteristic chemical reaction of that compound. The same function group will behave similarly, by undergoing similar reactions, regardless of the compound of which it is a part. The atoms or the group of atoms by which the characteristics reactions of organic compounds are determined, that atom or group of atoms is called a functional group.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Frequently Asked Questions (FAQs)
Functional are those having special activities or tasks. In chemistry, it is the functional group which when attached to a particular molecule will change its physical and chemical properties.
R is an abbreviation used to represent hydrocarbon that is compounds containing carbon and hydrogen. R1, R2, R3, etc. is also used to represent different hydrocarbon groups.
A particular group of atoms or substituent which when attached to a molecule will be responsible for the characteristic properties of that molecule. Amines, alcohols, anhydrides, acid chlorides, haloalkanes are some of the common examples.
Carbonyl is the functional group present in amides.
A series of compounds having similar chemical properties and same functional group are called homologous series.
Hydroxyl group.