Galvanic Cell - Voltaic Cell, Definition, Principle, Diagram with FAQs
What is a galvanic cell?
A cell is an electrochemical cell that converts the energy of spontaneous redox reactions into power. Galvanic cell can be a mode of electrochemical cell where oxidation takes place. A cell is an electrochemical cell that generates electricity through chemical processes. Let's have a look at how a voltaic or cell is made.
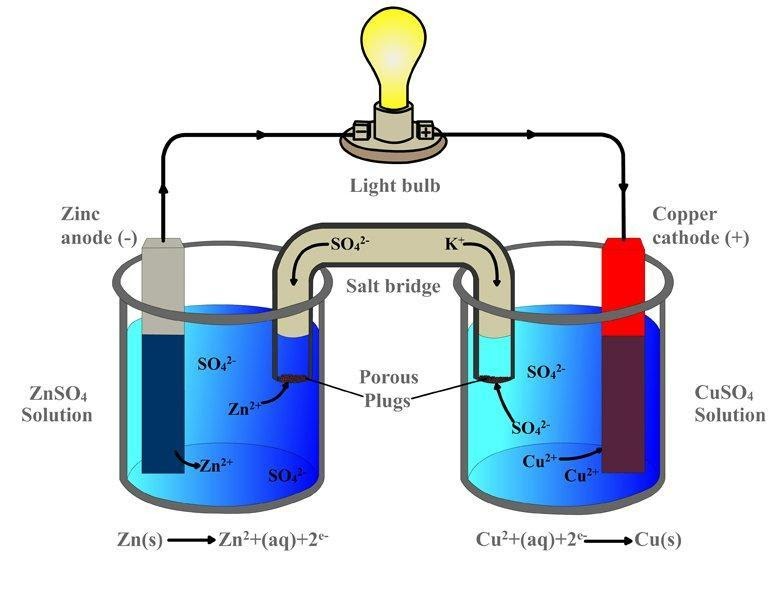
Electrochemical cell diagram
Electrons are transferred from one species to a special one in oxidation-reduction processes. If a reaction occurs spontaneously, energy is released. As a result, the liberated energy is put to good use. The reaction must be split into two half-reactions to deal with this energy: oxidation and reduction. The primary cell reactions are put into two distinct containers with wire so on maneuver the electrons from one end to the other.
These land up within the formation of an electrical device. Galvanic cells have typically been employed as DC power sources. An easy electric cell may contain simply one electrolyte separated by a semipermeable membrane, or it's visiting have two half-cells in an exceedingly very more complicated variant.
The salt bridge is created of an inert electrolyte, like potassium sulphate, whose ions flow into the individual half-cells to balance the fees that are built up at the electrodes. The mnemonic "Red Cat an Ox'' states that oxidation takes place at the anode and reduction takes place at the cathode. The anode is the negative terminal for the first cell current because the reaction at the anode is the source of electrons for this.
Also read -
Principle of Galvanic (Voltaic) Cell
The Gibbs energy of spontaneous redox reaction within the first cell is usually in command of the craft done by an electric cell. In most situations, it consists of two half cells and a salt bridge. Each half cell also includes a metallic electrode submerged in an electrolyte. These two half cells are externally connected to a voltmeter and a switch in metallic wires. When both electrodes are submerged within the identical electrolyte, a salt bridge isn't usually required.
Diagram of cell (voltaic cell)
Related Topics Link, |
working of galvanic (voltaic) cell:
The working of an electric cell is relatively uncomplicated. It includes an action that produces power as a by-product. A cell uses the energy transfer between electrons to convert energy into electric energy during a redox reaction. The ability to separate the flow of electrons within the method of oxidation and reduction, generating a half reaction, and linking each with a wire so a conduit for the flow of electrons through such wire is produced, is used during a cell.
A current is defined because of the flow of electrons. A current of this type is created to flow via a wire so on complete a circuit and procure an output in any device, sort of television or a watch. A primary cell could also be made of any two metals. If these two metals inherit contact with each other, they'll create the anode and cathode. This mix permits more anoxic metals to experience galvanic corrosion.
In a cell, when an electrode is exposed to the electrolyte at the electrode-electrolyte interface, the metal electrode's atoms tend to supply ions within the electrolyte solution, leaving the electrons at the electrode behind. The metal electrode becomes charged as a result. On the other hand, metal ions within the electrolyte solution have a bent to decide on a metal electrode. The electrode becomes charged as a result.
Charge separation is observed under equilibrium conditions, and also the electrode is often positively or charged counting on the inclinations of two opposing reactions.
Galvanic Cell parts Anode - This electrode is where oxidation takes place. The reduction takes place at the cathode electrode. Salt bridge - A salt bridge contains the electrolytes needed to complete the circuit in a very cell.
Reduction and oxidation reactions are segregated into compartments in half-cells. External circuit - Allows electrons to travel freely between electrodes. A load might be a component during a circuit that relies on the flow of electrons to accomplish its function.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 3 Electrochemistry
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3 Electrochemistry
- NCERT notes Class 12 Chemistry Chapter 3 Electrochemistry
Constructing a cell
To make a galvanic cell, you will have to follow the steps below.
Two electrodes would be great for the cell. The cathode, a charged electrode, are one altogether these electrodes, while the anode, a charged electrode, are visiting the other. The galvanic cell's two fundamental components are these two electrodes. The cathode should perform the reduction half-reaction, whereas the anode should perform the oxidation half-reaction.
Any two metals are going to be utilized to induce the reaction, as previously stated. Cell Example More than a century ago, electrochemical or galvanic cells were introduced as how for researching the thermodynamic features of fused salts. A galvanic cell, like Daniel's cell, turns energy into power.
In Daniel's cell, copper ions are reduced at the cathode, while zinc is oxidized at the anode. Galvanic cell reaction/ Daniel cell reactions at the cathode and anode are as follows:
Cathode: Cu 2+ + 2e–
Anode: Zn2+ + 2e–
The following are a variety of the key phrases that are utilized in galvanic cells: The two metals that operate because the cathode and anode are named as phase boundaries. The connecting bridge or medium that permits a redox reaction to need place is known as a salt bridge. The chemical processes that allow an electrical current to come back up with and flow through a cell are oxidation and reduction.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: